| Main idea of our research |
|---|
| Physiologists always ask How does it work |
Our main research topic is the tight junction: |
|
| ... and the transporters of the cell membranes: |
|
| Our research is mirrored in physiology textbook chapters: |
|
![]()
 |
|
Tight junction proteins comprise three (even four if one includes JAM) families. All are transmembrane proteins having one important feature in common: They are arranged in such a way that they interact with other TJ proteins of the neighboring cell and by this are able to seal the cleft between these cells against unlimited passage of solutes and water.
Two of these families possess four transmembrane domains (tetraspan proteins).
Most claudins and TAMPs act as barrier formers within the TJ. In contrast, some claudins form paracellular channels through the TJ which are selective for cations (claudin-2, claudin-10b, claudin-15), anions (claudin-10a, claudin-17) or water (claudin-2).
All tetraspan TJ proteins comprise intracellular N- and C-terminals, one small intracellular loop, and two extracellular loops (ECL1 and -2). ECL1 is thought to determine the paracellular barrier and/or channel function. ECL2 may act as mechanical contact between opposing tight junction proteins. The exact molecular structure of some proteins is partly to almost fully resolved.
Another two families of TJ proteins contain one transmembrane domain:
Important functions are mediated by numerous intracellularly located proteins (which therefore are not counted as TJ components in a strict sense).
Intracellular proteins like ZO1 and ZO2 connect many of the claudins and the TAMPs the actin cytoskeleton and are therefore termed scaffold proteins.
"Berlin TJ conference" volumes: I, 2009
 II, 2012
II, 2012
 III, 2017
III, 2017
 IV: 2022/2023
IV: 2022/2023
Actual reviews on TJ proteins
Günzel D, Lehmann M, Honigmann A (2026) Tight junction structure, assembly, and (dys)function. Nat. Rev. Mol. Cell. Biol. (accepted 12.01.2026)
Citi S, Fromm M, Furuse M, Gonzalez-Mariscal L, Nusrat A, Tsukita S, Turner JR (2024) A short guide to the tight junction. J. Cell Sci. 137(9): jcs261776 (12 pages), doi: 10.1242/jcs.261776
Meoli L, Günzel D (2023) The role of claudins in homeostasis. Nat. Rev. Nephrol.
Meoli L, Günzel D (2020) Channel functions of claudins in the organization of biological systems. BBA - Biomembranes 1862(9): 183344 (18 pages) (°IF 3.8) [Part of Special Volume "The vertebrate epithelial apical junctional complex", Hervé JC ed.] [PubMed] [WebPage] [PDF] [Suppl. Fig. S1]
Piontek J, Krug SM, Protze J, Krause G, Fromm M (2020) Molecular architecture and assembly of the tight junction backbone. BBA - Biomembranes 1862(7): 183279 (15 pages) (°IF 3.8) [Part of Special Volume "The vertebrate epithelial apical junctional complex", Hervé JC ed.], doi: 10.1016/j.bbamem.2020.183279
Barmeyer C, Fromm M, Schulzke JD (2017) Active and passive involvement of claudins in the pathophysiology of intestinal inflammatory diseases. Pflügers Arch. 469: 15-26 [PubMed] [WebPage] [PDF]
Fromm M, Piontek J, Rosenthal R, Günzel D, Krug SM (2017) Tight junctions of the proximal tubule and their channel proteins. Pflügers Arch. 469: 877-887 [PubMed] [WebPage] [PDF]
Günzel D (2017) Claudins: Vital partners in trans- and paracellular transport coupling. Pflügers Arch. 469(1): 35-44 [PubMed] [WebPage] [PDF]
Classics
Krug SM, Schulzke JD, Fromm M (2014) Tight junction, selective permeability, and related diseases. Semin. Cell Devel. Biol. 36: 166-176 [PubMed] [WebPage] [PDF] (Review)
Günzel D, Fromm M (2012) Claudins and other tight junction proteins. Compreh. Physiol. (former Handbook of Physiology) 2(3): 1819-1852 [PubMed] [WebPage] [PDF] (Handbook article)
Günzel D, Yu AS (2012) Claudins and the modulation of tight junction permeability. Physiol. Rev. 93(2): 525-569 [PubMed] [WebPage] [PDF] (Review)
Special Issues
Fromm M, Krug SM, eds. (2024) The tight junction and its proteins: From structure to pathologies. Special Issue of Int. J. Mol. Sci. (15 papers) Collection of papers
Krug SM, ed. (2024) Solving the puzzle: Molecular research in inflammatory bowel diseases, 2nd edition. Special Issue of
Bücker R, ed. (2023) Enterotoxins and mucosal pathomechanisms. Special Issue of Toxins (Basel) (5 papers) Collection of papers
Krug SM, ed. (2023) Solving the puzzle: Molecular research in inflammatory bowel diseases. Special Issue of Int. J. Mol. Sci. (12 papers) Collection of papers, doi: 10.3390/books978-3-0365-9863-5
Fromm M, Krug SM, eds. (2020) The tight junction and Its proteins: More than just a barrier, Special Issue of Int. J. Mol. Sci. Volumes 1 and 2. (44 papers):
Fromm M, Günzel D, Schulzke JD, Issue Editors (2017) Tight junctions and their proteins, I. Ann. N.Y. Acad. Sci. 1397: 1-230 [Contents w. links to papers]
Schulzke JD, Günzel D, Fromm M, Issue Editors (2017) Tight junctions and their proteins, II. Ann. N.Y. Acad. Sci. 1405: 1-214 [Contents w. links to papers]
![]()
Pouyiourou I, Fromm A, Piontek J, Rosenthal R, Furuse M*, Günzel D* (*shared last authorship) (2025) Ion permeability profiles of renal paracellular channel-forming claudins. Acta Physiol. 241: e14264 (18 pages). doi: 10.1111/apha.14264
Meoli L, Günzel D (2020) Channel functions of claudins in the organization of biological systems. BBA - Biomembranes 1862(9): 183344 (18 pages) (°IF 3.8) [Part of Special Volume "The vertebrate epithelial apical junctional complex", Hervé JC ed.] [PubMed] [WebPage] [PDF] [Suppl. Fig. S1]
Günzel D (2017) Claudins: vital partners in trans- and paracellular transport coupling. Pflügers Arch. 469(1): 35-44 [PubMed] [WebPage] [PDF] (Review)
Günzel D, Yu AS (2012) Claudins and the modulation of tight junction permeability. Physiol. Rev. 93(2): 525-569 [PubMed] [WebPage] [PDF] (Review)
Günzel D, Fromm M (2012) Claudins and other tight junction proteins. Compreh. Physiol. (former Handbook of Physiology) 2(3): 1819-1852 [PubMed] (Handbook article)
Kirschner N*, Rosenthal R* (*shared first authorship), Furuse M, Moll I, Fromm M, Brandner JM (2013) Contribution of tight junction proteins to ion, macromolecule, and water barrier in keratinocytes. J. Invest. Dermatol. 133(5): 1161-1169 [PubMed] [WebPage] [PDF] [Supplement]
Hackel D, Krug SM, Sauer RS, Mousa SA, Böcker A, Pflücke D, Wrede EJ, Kistner K, Hoffmann T, Niedermirtl B, Sommer C, Bloch L, Huber O, Blasig IE, Amasheh S, Reeh PW, Fromm M, Brack A, Rittner HL (2012) Transient opening of the perineurial barrier for analgesic drug delivery. Proc. Natl. Acad. Sci. USA 109(29): E2018-E2027 [PubMed] [WebPage] [PDF]
Kirschner N, Houdek P, Fromm M, Moll I, Brandner JM (2010) Tight junctions form a barrier in human epidermis. Eur. J. Cell Biol. 89(11): 839-842 [PubMed] [WebPage] [PDF]
Tebbe B, Mankertz J, Schwarz C, Amasheh S, Fromm M, Schultz-Ehrenburg U, Sánchez Ruderisch H, Schulzke JD, Orfanos CE (2002) Tight junction proteins: A novel class of integral membrane proteins. Expression in human epidermis and HaCaT keratinocytes. Arch. Dermatol. Res. 294: 14-18 [PubMed] [WebPage] [PDF]
Martínez-Perafán F, Fromm A, van der Veen RE, Waldow A, Lehmann M, Krug SM, Günzel D, Rosenthal R, Fromm M, Piontek J (2025) Effect of claudin-1 or -3 expression on cation and water channel properties of claudin-2.
Breiderhoff T*, Himmerkus N* (*shared first authorship), Meoli L, Fromm A, Sewerin S, Kriuchkova N, Nagel O, Ladilov Y, Krug SM, Quintanova C, Stumpp M, Garbe-Schönberg D, Westernströer U, Merkel C, Brinkhus MA, Altmüller J, Schweiger MR, Müller D, Mutig K, Morawski M, Halbritter J, Milatz S, Bleich M*, Günzel D* (*shared last authorship) (2022) Claudin-10a deficiency shifts proximal tubular Cl- permeability to cation selectivity via claudin-2 redistribution. J. Am. Soc. Nephrol. 33(4): 699-717, doi: 10.1681/ASN.2021030286
Fromm M, Piontek J, Rosenthal R, Günzel D, Krug SM (2017) Tight junctions of the proximal tubule and their channel proteins. Pflügers Arch. 469(7-8): 877-887 [PubMed] [WebPage] [PDF]
Rosenthal R, Czichos C, Theune D, Günzel D, Schulzke JD, Fromm M (2017) Water channels and barriers formed by claudins. Ann. N.Y. Acad. Sci.
Rosenthal R, Günzel D, Krug SM, Schulzke JD, Fromm M, Yu ASL (2017) Claudin-2-mediated cation and water transport share a common pore. Acta Physiol. 219(2): 521-536 [PubMed] [WebPage] [PDF]
Luettig J, Rosenthal R, Barmeyer C, Schulzke JD (2014) Claudin-2 as mediator of leaky gut barrier during intestinal inflammation. Tiss. Barriers 3(1-2): e977176, Invited Review, Special Issue "Tissue Barriers in Inflammation" [PubMed] [WebPage] [PDF] (Review)
Rosenthal R, Fromm M (2014) Significant water absorption goes paracellular in kidney proximal tubules. Am. J. Physiol. Renal Physiol. 306(1): F51-F52 [PubMed] [WebPage] [PDF] [Invited Editorial]
Rosenthal R, Milatz S, Krug SM, Oelrich B, Schulzke JD, Amasheh S, Günzel D, Fromm M (2010) Claudin-2, a component of the tight junction,
forms a paracellular water channel.
J. Cell Sci. 123(11): 1913-1921 [PubMed] [WebPage]
[PDF] [Supplement]
![]()
Doctoral thesis, medicine: Dr. med. Beibei Oelrich (2009) Entwicklung und Etablierung einer Methode zur Messung des epithelialen Wassertransports an Claudin-exprimierenden MDCK-Zellen. Magna cum laude
Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, Fromm M (2002) Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 115(24): 4969-4976 [PubMed] [WebPage] [PDF]
Yu ASL, Cheng MH, Angelow S, Günzel D, Kanzawa SA, Schneeberger EE, Fromm M, Coalson RD (2009) Molecular basis for cation selectivity in claudin-2-based paracellular pores: Identification of an electrostatic interaction site. J. Gen. Physiol. 133(1):111-127 [PubMed] [WebPage] [PDF]
Mankertz J*, Amasheh M* (*shared first authorship), Krug SM, Fromm A, Amasheh S, Hillenbrand B, Tavalali S, Fromm M, Schulzke JD (2009) Tumour necrosis factor alpha up-regulates claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol 3-kinase signaling. Cell Tiss. Res. 336(1): 67-77 [PubMed] [WebPage] [PDF]
Zeissig S, Bürgel N, Günzel D, Richter JF, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD (2007) Changes in expression and distribution of claudin-2, -5 and -8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut 56(1): 61-72 [PubMed] [WebPage] [PDF]
Mankertz J*, Hillenbrand B* (*shared first authorship), Tavalali S, Huber O, Fromm M, Schulzke JD (2004) Functional crosstalk between Wnt signaling and Cdx-related transcriptional activation in the regulation of the claudin-2 promoter activity. Biochem. Biophys. Res. Comm. 314(4): 1001-1007 [PubMed] [WebPage] [PDF]
Bürgel N, Bojarski C, Mankertz J, Zeitz M, Fromm M, Schulzke JD (2002) Mechanisms of diarrhea in collagenous colitis.
Gastroenterology
123(2): 433-443 [PubMed] [WebPage]
[PDF]
Claudin-3
Localization: Typical for tight epithelia
Function: We have characterized claudin-3 to be a general barrier former as it reduces permeability for ions without charge preference and uncharged solutes.
Clinical Impact: Claudin-3 and -4 are receptors for the enterotoxin of Clostridium perfringens.
Hempel C, Protze J, Altun E, Riebe B, Piontek A, Fromm A, Lee IM, Saleh T, Günzel D, Krause G, Piontek J (2020) Assembly of tight junction strands: Claudin-10b and claudin-3 form homo-tetrameric building blocks that polymerize in a channel-independent manner. J. Mol. Biol. 432(7): 2405-2427 (°IF 5.1) [PubMed] [WebPage] [PDF] [Supplementary Figures S1-S11] [Supplementary Table S1]
Milatz S*, Himmerkus N* (*shared first authorship), Wulfmeyer VC, Drewell H, Mutig K, Hou J, Breiderhoff T, Müller D, Fromm M, Bleich M°, Günzel D° (°shared last authorship) (2017) Mosaic expression of claudins in thick ascending limbs of Henle results in spatial separation of paracellular Na+ and Mg2+ transport. Proc. Natl. Acad. Sci. USA 114(2): E219-E227 [PubMed] [WebPage] [PDF+Supplement]. "Paper of the month" 03/2017 of the German Physiological Society
Milatz S, Krug SM, Rosenthal R, Günzel D, Müller D, Schulzke JD, Amasheh S*, Fromm M* (*shared last authorship) (2010)
Claudin-3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes. Biochim. Biophys. Acta Biomembr. [PubMed]
[WebPage] [PDF]
Doctoral thesis, biology: Dr. rer. nat. Susanne Milatz (2010)
Funktionelle Charakterisierung des Tight Junction-Proteins Claudin-3 in Epithel- und Endothelzellen.
Magna cum laude
Markov AG, Falchuk EL, Kruglova NM, Rybalchenko OV, Fromm M, Amasheh S (2014) Comparative analysis of theophylline and cholera toxin in rat
colon reveals an induction of sealing tight junction proteins. Pflügers Arch. 466(11): 2059-2065 [PubMed]
[WebPage] [PDF]
Tebbe B, Mankertz J, Schwarz C, Amasheh S, Fromm M, Schultz-Ehrenburg U, Sánchez Ruderisch H, Schulzke JD, Orfanos CE (2002) Tight junction proteins: A novel
class of integral membrane proteins. Expression in human epidermis and HaCaT keratinocytes.
Arch. Dermatol. Res. 294: 14-18 [PubMed] [WebPage]
[PDF]
Claudin-4
Localization: Typical for tight epithelia
Function: Paracellular barrier
Clinical Impact: In the Williams-Beuren-Syndrome, claudin-4 is not expressed .
van der Veen RE, Piontek J, Bieck M, Saiti A, Gonschior H, Lehmann M (2024) Claudin-4 polymerizes after a small extracellular claudin-3-like substitution.
J. Biol. Chem. 300(10): 107693 (14 pages). doi: 10.1016/j.jbc.2024.107693Eichner M, Augustin C, Fromm A, Piontek A, Walther W, Bücker R, Fromm M, Krause G, Schulzke JD, Günzel D, Piontek J (2018) In colon epithelia, Clostridium perfringens enterotoxin causes focal leaks by targeting claudins which are apically accessible due to tight junction derangement. J. Infect. Dis. 217(1): 147-157 [PubMed] [WebPage] [PDF]
Markov AG, Falchuk EL, Kruglova NM, Rybalchenko OV, Fromm M, Amasheh S (2014) Comparative analysis of theophylline and cholera toxin in rat colon reveals an induction of sealing tight junction proteins. Pflügers Arch. 466(11): 2059-2065 [PubMed] [WebPage] [PDF]
Amasheh M, Schlichter S, Amasheh S, Mankertz J, Zeitz M, Fromm M, Schulzke JD (2008) Quercetin enhances epithelial barrier function and increases claudin-4 expression in Caco-2 cells. J. Nutr. 138(6): 1067-1073 [PubMed] [WebPage] [PDF]
Florian P, Amasheh S, Lessidrensky M, Todt I, Bloedow A, Ernst A, Fromm M, Gitter AH (2003) Claudins in the tight junctions of stria vascularis marginal cells. Biochem. Biophys. Res. Comm. 304: 5-10 [PubMed] [WebPage] [PDF]
Claudin-5
Localization: Typical for endothelia
Function: We were able to show that claudin-5 belongs to the barrier-forming claudins and that it is expressed also in some epithelia.
Clinical Impact: Claudin-5 is deleted in patients suffering from velo-cardio-facial syndrome (DiGeorge syndrome). Claudin-5-deficient mice exhibit a barrier defect
of the blood-brain barrier.
Barmeyer C, Erko I, Awad K, Fromm A, Bojarski C, Meissner S, Loddenkemper C, Kerick M, Siegmund B, Fromm M, Schweiger MR, Schulzke JD (2017) Epithelial barrier dysfunction in lymphocytic colitis through cytokine-dependent internalization of claudin-5 and -8. J. Gastroenterol. 52(10): 1090-1100 [PubMed] [WebPage] [PDF] [Supplement]
Protze J, Eichner M, Piontek A, Dinter S, Rossa J, Blecharz KG, Vajkoczy P, Piontek J*, Krause G* (*shared last authorship) (2014) Directed structural modification of Clostridium perfringens enterotoxin to enhance binding to claudin-5. Cell. Mol. Life Sci. 72(7): 1417-1432 [PubMed] [WebPage] [PDF]
Dittmann I, Amasheh M, Krug SM, Markov AG, Fromm M, Amasheh S (2014) Laurate permeabilizes the paracellular pathway for small molecules in the intestinal epithelial cell model HT-29/B6 via opening the tight junctions by reversible relocation of claudin-5. Pharm. Res. 31(9): 2539–2548 [PubMed] [WebPage] [PDF]
Claudin-6
Claudin-7
Claudin-8
Nattramilarasu PK, Bücker R, Lobo de Sá FD, Fromm A, Nagel O, Lee IM, Butkevych E, Mousavi S, Genger C, Kløve S, Heimesaat MM, Bereswill S, Schweiger MR, Nielsen HL, Troeger H, Schulzke JD (2020) Campylobacter concisus impairs sodium absorption in colonic epithelium via ENaC dysfunction and claudin-8 disruption. Int. J. Mol. Sci. (Special Issue "Ion and molecule transport in membrane systems 2.0") 21(2): e373 (23 pages) (°IF 4.2) [PubMed] [WebPage] [PDF] [Supplements]
Barmeyer C, Erko I, Awad K, Fromm A, Bojarski C, Meissner S, Loddenkemper C, Kerick M, Siegmund B, Fromm M, Schweiger MR, Schulzke JD (2017) Epithelial barrier dysfunction in lymphocytic colitis through cytokine-dependent internalization of claudin-5 and -8. J. Gastroenterol. 52(10): 1090-1100 [PubMed] [WebPage] [PDF] [Supplement]
Amasheh S*, Milatz S* (*shared first authorship), Krug SM, Bergs M, Amasheh M, Schulzke JD, Fromm M (2009) Na+ absorption defends from paracellular back-leakage by claudin-8 upregulation. Biochem. Biophys. Res. Comm. 378: 45-50 [PubMed] [WebPage] [PDF]
Claudin-
9Nagarajan SK, Klein S, Fadakar BS, Piontek J (2023) Claudin-10b cation channels in tight junction strands: Octameric-interlocked pore barrels constitute paracellular channels with low water permeability
Quintanova C*, Himmerkus N* (*shared first authorship), Svendsen SL, von Schwerdtner O, Merkel C, Pinckert L, Mutig K, Breiderhoff T, Müller D, Günzel D, Bleich M (2022) Unrecognized role of claudin-10b in basolateral membrane infoldings of the thick ascending limb. Ann. NY Acad. Sci. 1517(1): 266-278, doi: org/10.1111/nyas.14882
Hempel C*, Rosenthal R* (*shared first authorship), Fromm A, Krug SM, Fromm M, Günzel D, Piontek J (2022) Tight junction channels claudin-10b and claudin-15: Functional mapping of pore-lining residues. Ann. NY Acad. Sci. 1515(1): 129-142, doi: 10.1111/nyas.14794
Breiderhoff T*, Himmerkus N* (*shared first authorship), Meoli L, Fromm A, Sewerin S, Kriuchkova N, Nagel O, Ladilov Y, Krug SM, Quintanova C, Stumpp M, Garbe-Schönberg D, Westernströer U, Merkel C, Brinkhus MA, Altmüller J, Schweiger MR, Müller D, Mutig K, Morawski M, Halbritter J, Milatz S, Bleich M*, Günzel D* (*shared last authorship) (2022) Claudin-10a deficiency shifts proximal tubular Cl- permeability to cation selectivity via claudin-2 redistribution. J. Am. Soc. Nephrol. 33(4): 699-717, doi: 10.1681/ASN.2021030286
Sewerin S, Piontek J, Schönauer R, Grunewald S, Rauch A, Neuber S, Bergmann C, Günzel D*, Halbritter J* (*shared last authorship) (2021) Defective claudin-10 causes a novel variation of HELIX syndrome through compromised tight junction strand assembly. Genes Dis. [12.06.21 accepted]
Hempel C, Protze J, Altun E, Riebe B, Piontek A, Fromm A, Lee IM, Saleh T, Günzel D, Krause G, Piontek J (2020) Assembly of tight junction strands: Claudin-10b and claudin-3 form homo-tetrameric building blocks that polymerize in a channel-independent manner. J. Mol. Biol. 432(7): 2405-2427 [PubMed] [WebPage] [PDF] [Supplementary Figures S1-S11] [Supplementary Table S1]
Breiderhoff T*, Himmerkus N* (shared first authorship), Drewell H, Plain A, Günzel D, Mutig K, Willnow TE°, Müller D°, Bleich M° (°corresponding) (2017) Deletion of claudin-10 in the kidney rescues claudin-16-deficient mice from hypomagnesmia and hypercalciuria. Kidney Int. 93(3): 580-588 [PubMed] [WebSite] [PDF]
Klar J, Piontek J, Milatz S, Tariq M, Jameel M, Breiderhoff T, Schuster J, Fatima A, Asif M, Sher M, Mäbert K, Fromm A, Baig SM, Günzel D, Dahl N (2017) Altered paracellular cation permeability due to a rare CLDN10B variant causes anhidrosis and kidney damage. Plos Genet.
Fromm M, Piontek J, Rosenthal R, Günzel D, Krug SM (2017) Tight junctions of the proximal tubule and their channel proteins. Pflügers Arch. 469(7-8): 877-887 [PubMed] [WebPage] [PDF]
Milatz S, Piontek J, Hempel C, Meoli L, Grohe C, Fromm A, Lee IM, El-Athman R, Günzel D (2017) Tight junction strand formation by claudin-10 isoforms and claudin-10a/-10b chimeras. Ann. N.Y. Acad. Sci. 1405: 102-115 (°IF 4.7) [PubMed] [WebPage] [PDF]
Milatz S*, Himmerkus N* (*shared first authorship), Wulfmeyer VC, Drewell H, Mutig K, Hou J, Breiderhoff T, Müller D, Fromm M, Bleich M°, Günzel D° (°shared last authorship) (2017) Mosaic expression of claudins in thick ascending limbs of Henle results in spatial separation of paracellular Na+ and Mg2+ transport. Proc. Natl. Acad. Sci. USA 114(2): E219-E227 [PubMed] [WebPage] [PDF+Supplement]. "Paper of the month" 03/2017 of the German Physiological Society
Milatz S, Breiderhoff T (2017) One gene, two paracellular ion channels ‒ claudin-10 in the kidney. Pflügers Arch. 469(1): 115-121 [PubMed] [WebPage] [PDF] (Review)
Rosenthal R, Milatz S, Krug SM, Oelrich B, Schulzke JD, Amasheh S, Günzel D, Fromm M (2010) Claudin-2, a component of the tight junction, forms a
paracellular water channel.
J. Cell Sci. 123(11): 1913-1921 [PubMed] [WebPage]
[PDF] [Supplement]
![]()
Günzel D, Stuiver M, Kausalya PJ, Haisch L, Krug SM, Rosenthal R, Meij IC, Hunziker W, Fromm M, Müller D (2009) Claudin-10 exists in six alternatively spliced isoforms which exhibit distinct localization and function. J. Cell Sci. 122: 1507-1517 [PubMed] [WebPage] [PDF]
Claudin-11 (= OSP)
Localization: CNS: oligodendrocytes, Testis: Sertoli cells, Ear: organ of Corti, Kidney: prox. tubule, Henle's loop
Function: Barrier
Claudin-12
Localization: Microvascular cells in the brain, lung, intestine, sciatic nerve, and cancer cells
Function: Barrier, however in vitro increases calcium permeability and is upregulated by vitamin D
Claudin-12 is one of the few claudins without a PDZ binding motif.
Chen JT*, Hu X* (*shared first authorship), Otto IUC, Schürger C, Rogalla von Bieberstein B, Doppler K, Krug SM, Hankir MK, Blasig R, Sommer C, Brack A, Blasig IE, Rittner HL (2023) Myelin barrier breakdown, mechanical hypersensitivity, and painfulness in polyneuropathy with claudin-12 deficiency. Neurobiol. Dis. 30: 106246 (## pages), doi: 10.1016/j.nbd.2023.106246
Claudin-14
Localization: Ear: cochlea hair cells; Kidney: collecting duct
Function: Barrier in cochlea hair cells
Hempel C*, Rosenthal R* (*shared first authorship), Fromm A, Krug SM, Fromm M, Günzel D, Piontek J (2022) Tight junction channels claudin-10b and claudin-15: Functional mapping of pore-lining residues. Ann. NY Acad. Sci. 1515(1): 129-142, doi: 10.1111/nyas.14794
Rosenthal R, Günzel D, Piontek J, Krug SM, Ayala-Torres C, Hempel C, Theune D, Fromm M (2020) Claudin-15 forms a water channel through the tight junction
with distinct function compared to claudin-2. Acta Physiol. 228(1): e13334 (15 pages)
doi: 10.1111/apha.13334
- Editorial Commentary on this article: Alexander RT (2020) Claudin-15 is not a drag! Acta Physiol. 228(1): e13397,
doi: 10.1111/apha.13397
Claudin-16 (initially named paracellin-1)
Localization: Kidney: thick ascending limb of Henle's loop and distal tubule
Function: Claudin-16 (together with claudin-19) facilitates renal magnesium and calcium transport
Clinical Impact: Mutations of claudin-16 and claudin-19 are causative for familial hypomagnesemia, together with hypercalciuria
and nephrocalcinosis (FHHNC)
Breiderhoff T*, Himmerkus N* (shared first authorship), Drewell H, Plain A, Günzel D, Mutig K, Willnow TE°, Müller D°, Bleich M° (°corresponding) (2017) Deletion of claudin-10 in the kidney rescues claudin-16-deficient mice from hypomagnesmia and hypercalciuria. Kidney Int. 93(3): 580-588 [PubMed] [WebSite] [PDF]
Milatz S*, Himmerkus N* (*shared first authorship), Wulfmeyer VC, Drewell H, Mutig K, Hou J, Breiderhoff T, Müller D, Fromm M, Bleich M°, Günzel D° (°shared last authorship) (2017) Mosaic expression of claudins in thick ascending limbs of Henle results in spatial separation of paracellular Na+ and Mg2+ transport. Proc. Natl. Acad. Sci. USA 114(2): E219-E227 [PubMed] [WebPage] [PDF+Supplement]. "Paper of the month" 03/2017 of the German Physiological Society
Will C, Breiderhoff T, Thumfart J, Stuiver M, Kopplin K, Sommer K, Günzel D, Querfeld U, Meij IC, Shan Q, Bleich M, Willnow TE, Müller D (2010) Targeted deletion of murine Cldn16 identifies extra- and intrarenal compensatory mechanisms of Ca2+ and Mg2+ wasting. Am. J. Physiol. Renal Physiol. 298: F1152-F1161 [PubMed] [WebPage] [PDF]
Günzel D, Amasheh S, Pfaffenbach S, Richter JF, Kausalya PJ, Hunziker W, Fromm M (2009) Claudin-16 affects transcellular Cl- secretion in MDCK cells. J. Physiol. (Lond.) 587(15): 3777-3793 [PubMed] [WebPage] [PDF] [Supplement]
Kausalya PJ*, Amasheh S* (*shared first authorship, p 890), Günzel D, Wurps H, Müller D, Fromm M, Hunziker W (2006) Disease-associated mutations affect intracellular traffic and paracellular Mg2+ transport function of claudin-16. J. Clin. Invest. 116(4): 878-891
Claudin-17
Localization: Kidney: abundant in the nephron. Marginal in brain.
Function: Claudin-17 forms paracellular anion channels (discovered by Krug et al. 2012, Cell. Mol. Life Sci.).
Molecular: Claudin-17 anion selectivity critically depends on a positive charge at position 65.
Fromm M, Piontek J, Rosenthal R, Günzel D, Krug SM (2017) Tight junctions of the proximal tubule and their channel proteins. Pflügers Arch. 469(7-8): 877-887 [PubMed] [WebPage] [PDF]
Conrad MP*, Piontek J* (*shared first authorship), Günzel D, Fromm M, Krug SM (2016) Molecular basis of claudin-17 anion selectivity. Cell. Mol. Life Sci. 73(1): 185-200
Krug SM, Günzel D, Conrad MP, Rosenthal R, Fromm A, Amasheh S, Schulzke JD, Fromm M (2012) Claudin-17 forms tight junction channels with distinct anion selectivity. Cell. Mol. Life Sci. 69(16): 2765-2778 [PubMed] [WebPage] [PDF] [Supplement]
Claudin-18
Localization: Cochlea, Corti organ, Stria vascularis marginal cells; Claudin 18-1:
Lung; Claudin-18-2: Stomach, Oesophagus, also small intestine
Claudin-19
Localization: Kidney: thick ascending limb of Henle's loop and distal nephron,
Nerve: Schwann cells
Function: Claudin-19 (together with claudin-16) facilitates renal magnesium and calcium transport
Clinical Impact: Mutations of claudin-16 and claudin-19 are causative for familial hypomagnesemia, together with hypercalciuria
and nephrocalcinosis (FHHNC)
Milatz S*, Himmerkus N* (*shared first authorship), Wulfmeyer VC, Drewell H, Mutig K, Hou J, Breiderhoff T, Müller D, Fromm M, Bleich M°, Günzel D° (°shared last authorship) (2017) Mosaic expression of claudins in thick ascending limbs of Henle results in spatial separation of paracellular Na+ and Mg2+ transport. Proc. Natl. Acad. Sci. USA 114(2): E219-E227 [PubMed] [WebPage] [PDF+Supplement]. "Paper of the month" 03/2017 of the German Physiological Society
Claudin-20
Localization: Eye: Retina pigment epithelium
Claudin-21
Expressed in the embryonic state only.
Function: May act as more or less unspecific channel (Tanaka et al. 2016, Mol. Cell Biol.)
Claudin-22
Localization: Choroid plexus (Kratzer et al. 2012, Histochem. Cell Biol.)
Claudin-23
Localization: Skin, placenta, stomach, colon tumors
Claudin-24
Claudin-25
Claudin-26 (= TMEM 114)
Claudin-27
![]()
TAMP family
General: TAMP stands for Tight junction-Associated Marvel Proteins. The TAMP family includes occludin, tricellulin, and marvelD3, which
share a transmembrane domain motif called MARVEL (Myelin and lymphocyte And Related protein for VEsicle trafficking and membrane Link).
Occludin
Localization: All epithelia
Function: The function of occludin is still poorly understood. In a collaboration, the lab of Shoichiro Tsukita and our group have shown that in occludin-KO mice the tight juncion barrier
is unaltered. This means that occludin either has no intrinsic barrier properties or can be replaced by other components of the tight junction.
Saitou M, Furuse M, Sasaki H, Schulzke JD, Fromm M, Takano H, Noda T, Tsukita S (2000) Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol. Biol. Cell 11(12): 4131-4142 [PubMed] [WebPage] [PDF]
In occludin-knockout mice the glandular structure of the stomach exhibited a complete loss of parietal cells and mucus cell hyperplasia, as a result of which acid secretion was virtually abolished. A dramatic change in gastric morphology and secretory function indicates that occludin is involved in gastric epithelial differentiation.
Schulzke JD, Gitter AH, Mankertz J, Spiegel S, Seidler U, Amasheh S, Saitou M, Tsukita S, Fromm M (2005) Epithelial transport and barrier function in occludin-deficient mice. Biochim. Biophys. Acta - Biomembranes 1669(1): 34-42 [PubMed] [WebPage] [PDF]
Little is known about the regulatory mechanisms of occludin that influence occludin gene expression. We aimed to identify the sequences essential in cis for genomic regulation of tight junction
formation and to investigate their functional role in cytokine-dependent tight junction regulation.
Using genome walking cloning of occludin-specific human genomic DNA sequences, a 1853 bp DNA fragment containing the transcription start point of occludin cDNA sequences was amplified and sequenced.
The proinflammatory cytokines, TNFa
and interferon g diminished occludin promoter activity alone and even synergistically, suggesting a genomic regulation of alterations of the paracellular barrier. Both
cytokines downregulate the expression of occludin, paralleling the barrier disturbance detected electrophysiologically. This could be an important mechanism in gastrointestinal diseases accompanied by
barrier defects, for example inflammatory bowel diseases.
Dörfel MJ, Westphal JK, Bellmann C, Krug SM, Cording J, Mittag S, Tauber R, Fromm M, Blasig IE, Huber O (2013) CK2-dependent phosphorylation of occludin regulates the interaction with ZO-proteins and tight junction integrity. Cell Commun. Signal.
Mankertz J, Waller JS, Hillenbrand B, Tavalali S, Florian P, Schöneberg T, Fromm M, Schulzke JD (2002) Gene expression of the tight junction protein occludin includes differential splicing and alternative promoter usage. Biochem. Biophys. Res. Comm. 298: 657-666 [PubMed] [PDF]
Mankertz J, Tavalali S, Schmitz H, Mankertz A, Fromm M, Schulzke JD (2000) Expression from the human occludin promoter is affected by tumor necrosis factor a and interferon g. J. Cell Sci. 113(Pt 11): 2085-2090 [PubMed] [PDF] [GenBank: Homo sapiens occludin gene, partial sequence]
Tricellulin (= marvelD2)
Localization: Tricellular tight junction (tTJ), i.e. the site where three epithelial or endothelial cells meet.
Function: Tricellulin was discovered by Shoichiro Tsukita who has died in Dec. 2005, a few days before his landmark paper appeared: Ikenouchi et al., 2005, J. Cell
Biol. 171(6): 939-945 [PubMed] [PDF].
In cell cultures, lack of tricellulin prevents the development of the epithelial barrier. We showed that tricellulin tightens the tricellular junction against macromolecules. We propose that, at
impaired tricellulin expression, the tTJ becomes a major site for the passage of macromolecules.
Ayala-Torres C, Krug SM, Schulzke JD, Rosenthal R*, Fromm M* (*shared last authorship) (2019) Tricellulin effect on paracellular water transport. Int. J. Mol. Sci. 20(22): 5700 (15 pages) (°IF 4.2) [PubMed] [WebPage] [PDF]
Krug SM, Bojarski C, Fromm A, Lee IM, Dames P, Richter JF, Turner JR, Fromm M*, Schulzke JD* (*shared last authorship) (2018) Tricellulin is regulated via interleukin-13-receptor α2, affects macromolecule uptake, and is decreased in ulcerative colitis. Mucosal Immunol. 11(2): 345-356
Krug SM, Hayaishi T, Iguchi D, Watari A, Takahashi A, Fromm M, Nagahama M, Takeda H, Okada Y, Sawasaki T, Doi T, Yagi K, Kondoh M (2017) Angubindin-1, a novel paracellular absorption enhancer acting at the tricellular tight junction. J. Contr. Release 260: 1-11 [PubMed] [WebPage] [PDF] [Supplement]
Schütz A, Radusheva V, Krug SM*, Heinemann U* (*shared last authorship) (2017) Crystal structure of the tricellulin C-terminal coiled-coil domain reveals a unique mode of dimerization. Ann. N.Y. Acad. Sci. 1405: 147-159
[PubMed] [WebPage] [PDF] [Supplement]Krug SM, Amasheh M, Dittmann I, Christoffel I, Fromm M, Amasheh S (2013) Sodium caprate as an enhancer of macromolecule permeation across tricellular tight junctions of intestinal cells. Biomaterials 34(1): 275-282 [PubMed] [WebPage] [PDF]
Krug SM, Amasheh S, Richter JF, Milatz S, Günzel D, Westphal JK, Huber O, Schulzke JD, Fromm M (2009) Tricellulin forms a barrier to
macromolecules in tricellular tight junctions without affecting ion permeability. Mol. Biol. Cell 20: 3713-3724 [PubMed]
[WebPage] [PDF] [Supplement
text] [Supplement video]
![]()
Doctoral thesis, biochemistry: Dr. rer. nat. Susanne M. Krug (2009) Tricellulin und seine Funktion in der trizellulären Tight Junction von Epithelzellen. Biochemie, FU Berlin. Summa cum laude
Westphal JK, Dörfel MJ, Krug SM, Cording JD, Piontek J, Blasig IE, Tauber R, Fromm M, Huber O (2010) Tricellulin forms homomeric and heteromeric tight junctional complexes. Cell. Mol. Life Sci. 67(12): 2057-2068 [PubMed] [WebPage] [Supplement WebPage] [PDF] [Suppl. Fig. 1] [Suppl. Fig. 2]
MarvelD3
Localization: Splice variant 1 within the bicellular TJ; splice variant 2 within the
tricellular TJ.
Function:
Regulation: couples tight junctions to the JNK pathway to regulate cell behavior and survival.
![]()
The term "angulin" was introduced by Higashi et al. 2013 [PubMed], integrating a family of three
single-span proteins predominatly localized in the
tricellular tight junction (tTJ):
- angulin-1 = LSR, lipolysis-stimulated lipoprotein receptor,
- angulin-2 = ILDR1, immunoglobulin-like domain-containing receptor 1,
- angulin-3 = ILDR2, immunoglobulin-like domain-containing receptor 2.
Angulin-1 and angulin-2 are relevant for recruitment of tricellulin to the tricellular TJ. If defective or decreased, the lack of well-localized tricellulin causes opening of the tTJ barrier.
Ayala-Torres C, Krug SM, Rosenthal R*, Fromm M* (*shared last authorship) (2021) Angulin-1 (LSR) affects paracellular water transport, however only in tight epithelial cells. Int. J. Mol. Sci.
Krug SM, Hayaishi T, Iguchi D, Watari A, Takahashi A, Fromm M, Nagahama M, Takeda H, Okada Y, Sawasaki T, Doi T, Yagi K, Kondoh M (2017) Angubindin-1, a novel paracellular absorption enhancer acting at the tricellular tight junction. J. Contr. Release 260: 1-11 [PubMed] [WebPage] [PDF] [Supplement]
![]()
The JAM (Junctional Adhesion Molecule) family consists of 7 members: Three "classical" ones (JAM-A, JAM-B, JAM-C) and four "non-classical" ones (CAR, ESAM, JAM-J, JAM-4). For comprehensive review see Garrido-Urbani et al. 2014 [PubMed]).
JAM proteins are localized just "below" the TJ strands (meaning more to basal cell side) and provide mechanical adhesion between lateral membranes of neighboring cells. JAM molecules have no direct barrier function by itself, but if JAM cell-cell contacts are impaired the lateral cell membranes lose contact. Necessarily, adjacent TJ proteins also lose contact to each other and the paracellular barrier opens.
JAMs form cis- and trans-interactions with other JAMs. All JAMs contain PDZ motifs and bind to numerous intracellular parters.
![]()
Scaffold proteinS: ZO-1 et al.
Scaffold proteins provide an intracellular connection between most claudins and the TAMPs with the actin cytoskeleton. Best-known are ZO-1 and ZO-2 (Zonula Occludens-1 and -2). The name "Zonula occludens" suggests they are tight junction proteins, but in a strict sense they are not. They are located intracellularly and connected via PDZ domains with the claudins, TAMPs, and JAMs.
Haas AJ, Zihni C, Krug SM, Maraspini R, Otani T, Furuse M, Honigmann A, Balda MS*, Matter K* (*shared last authorship) (2022) Reciprocal regulation of cell mechanics and ZO-1 guides tight junction assembly and epithelial morphogenesis. Cells 11(23): 3775 (30 pages), doi: 10.3390/cells11233775
Dörfel MJ, Westphal JK, Bellmann C, Krug SM, Cording J, Mittag S, Tauber R, Fromm M, Blasig IE, Huber O (2013) CK2-dependent phosphorylation of occludin regulates the interaction with ZO-proteins and tight junction integrity. Cell Commun. Signal.
Schumann M, Günzel D, Buergel N, Richter JF, Troeger H, May C, Fromm A, Sorgenfrei D, Daum S, Bojarski C, Heyman M, Zeitz M, Fromm M, Schulzke JD (2012) Cell polarity-determining proteins Par-3 and PP-1 are involved in epithelial tight junction defects in celiac disease. Gut 61(2): 220-228 [PubMed] [WebPage] [PDF] [Supplements]
![]()
|
Molecular structure of single claudins
The first crystal structure of a claudin was published in 2014 by Suzuki et al. for claudin-15 [PubMed].
This was a major breakthrough, which then also allowed for homology studies on other members of the claudin family.
We have performed studies on the molecular structure of the following claudins:
- Claudin-3 (Rossa, Plöger et al. 2014; Milatz et al. 2015; Legere et al. 2024)
- Claudin-5 (Rossa, Protze et al. 2014; Rossa, Plöger et al. 2014)
- Claudin-17 (Conrad et al. 2016)
Nagarajan SK, Weber J, Roderer D, Piontek J (2025) C. perfringens enterotoxin-claudin pore complex: Models for structure, mechanism of pore assembly and cation permeability. Comp. Struct. Biotechnol. J. 27: 287-306. doi: 10.1016/j.csbj.2024.11.048
van der Veen RE, Piontek J, Bieck M, Saiti A, Gonschior H, Lehmann M (2024) Claudin-4 polymerizes after a small extracellular claudin-3-like substitution. J. Biol. Chem. 300(#): ###### (## pages). https://doi.org/10.1016/j.jbc.2024.107693
Legere EA, Baumholtz AI, Lachance JB, Archer M, Piontek J, Ryan AK (2024) Claudin-3 in the non-neural ectoderm is essential for neural fold fusion in chicken embryos. Dev. Biol. 507: 20-33. doi: 10.1016/j.ydbio.2023.12.009
Nagarajan SK, Piontek J (2024) Molecular dynamics simulations of claudin-10a and -10b ion channels: With similar architecture, different pore linings determine the opposite charge selectivity. Int. J. Mol. Sci. 25(6): 3161 (23 pages), doi: 10.3390/ijms25063161
Nagarajan SK, Klein S, Fadakar BS, Piontek J (2023) Claudin-10b cation channels in tight junction strands: Octameric-interlocked pore barrels constitute paracellular channels with low water permeability
Krause G, Protze J, Piontek J (2015) Assembly and function of claudins: Structure-function relationships based on homology models and crystal structures. Semin. Cell Devel. Biol. 42: 3-12 [PubMed] [WebPage] [PDF] (Review)
Piontek A, Rossa J, Protze J, Wolburg H, Hempel C, Günzel D, Krause G, Piontek J (2017) Polar and charged extracellular residues conserved among sealing claudins contribute to tight junction strand formation. Ann. N.Y. Acad. Sci.
![]()
Molecular architecture of tight junctions
Our comprehensive review on that topic:
Piontek J, Krug SM, Protze J, Krause G, Fromm M (2020) Molecular architecture and assembly of the tight junction backbone. BBA - Biomembranes 1862(7): 183279 (15 pages) (°IF 3.8) [Part of Special Volume "The vertebrate epithelial apical junctional complex", Hervé JC ed.] [PubMed] [WebPage] [PDF] [Supplement]
We have done studies on the oligomeric TJ-arrangement of the following claudins:
Barrier-forming claudins:
- Claudin-1 (Milatz et al. 2015; Piontek-A et al., 2017)
- Claudin-3 (Rossa, Plöger et al. 2014; Milatz et al. 2015; Piontek-A et al., 2017; Hempel et al. 2020)
Channel-forming claudins:
- Claudin-10a and claudin-10b (Milatz et al. 2017; Klar et al. 2017; Hempel et al. 2020; Hempel et al. 2022)
- Claudin-2, claudin-15, and claudin-17 (Krause et al. 2015; Fromm et al. 2017; Hempel et al. 2022)
Martínez-Perafán F, Fromm A, van der Veen RE, Waldow A, Lehmann M, Krug SM, Günzel D, Rosenthal R, Fromm M, Piontek J (2025) Effect of claudin-1 or -3 expression on cation and water channel properties of claudin-2.
Gonschior H, Schmied C, Van der Veen R, Eichhorst J, Himmerkus N, Piontek J, Günzel D, Bleich M, Furuse M, Haucke V, Lehmann M (2022) Nanoscale segregation of channel and barrier claudins enables paracellular ion flux. Nat. Commun. 13(1): 4985 (20 pages). doi: 10.1038/s41467-022-32533-4, Supplement
Hempel C*, Rosenthal R* (*shared first authorship), Fromm A, Krug SM, Fromm M, Günzel D, Piontek J (2022) Tight junction channels claudin-10b and claudin-15: Functional mapping of pore-lining residues. Ann. NY Acad. Sci. 1515(1): 129-142, doi: 10.1111/nyas.14794
Hempel C, Protze J, Altun E, Riebe B, Piontek A, Fromm A, Lee IM, Saleh T, Günzel D, Krause G, Piontek J (2020) Assembly of tight junction strands: Claudin-10b and claudin-3 form homo-tetrameric building blocks that polymerize in a channel-independent manner. J. Mol. Biol. 432(7): 2405-2427 (°IF 5.1) [PubMed] [WebPage] [PDF] [Supplementary Figures S1-S11] [Supplementary Table S1]
Conrad MP*, Piontek J* (*shared first authorship), Günzel D, Fromm M, Krug SM (2016) Molecular basis of claudin-17 anion selectivity. Cell. Mol. Life Sci. 73(1): 185-200 [PubMed] [WebPage] [PDF] [Supplement]
Eichner M, Protze J, Piontek A, Krause G, Piontek J (2017) Targeting and alteration of tight junctions by bacteria and their virulence factors such as Clostridium perfringens enterotoxin. Pflügers Arch. 469(1): 77-90 [PubMed] [WebPage] [PDF] (Review)
Fromm M, Piontek J, Rosenthal R, Günzel D, Krug SM (2017) Tight junctions of the proximal tubule and their channel proteins. Pflügers Arch. 469: 877-887 [PubMed] [WebPage] [PDF] (Review)
Klar J, Piontek J, Milatz S, Tariq M, Jameel M, Breiderhoff T, Schuster J, Fatima A, Asif M, Sher M, Mäbert K, Fromm A, Baig SM, Günzel D, Dahl N (2017) Altered paracellular cation permeability due to a rare CLDN10B variant causes anhidrosis and kidney damage. Plos Genet. 13(7): e1006897 (pages 1-22 + 6 Supplements) [PubMed] [WebPage] [PDF] [Supplement]
Krause G, Protze J, Piontek J (2015) Assembly and function of claudins: Structure-function relationships based on homology models and crystal structures. Semin. Cell Devel. Biol. 42: 3-12 [PubMed] [WebPage] [PDF] (Review)
Krause G, Winkler L, Müller SL, Haseloff RF, Piontek J, Blasig IE (2008) Structure and function of claudins. Biochim. Biophys. Acta 1778(3): 631-645 [PubMed] [WebPage] [PDF] (Review)
Milatz S, Piontek J, Schulzke JD, Blasig IE, Fromm M, Günzel D (2015) Probing the cis-arrangement of prototype tight junction proteins claudin-1 and claudin-3. Biochem. J. 468(3): 449-458 [PubMed] [WebPage] [PDF]
Piontek A, Rossa J, Protze J, Wolburg H, Hempel C, Günzel D, Krause G, Piontek J (2017) Polar and charged extracellular residues conserved among sealing claudins contribute to tight junction strand formation. Ann. N.Y. Acad. Sci. 1397: 143-156 [PubMed] [WebPage] [PDF]
Protze J, Eichner M, Piontek A, Dinter S, Rossa J, Blecharz KG, Vajkoczy P, Piontek J*, Krause G* (*shared last authorship) (2015) Directed structural modification of Clostridium perfringens enterotoxin to enhance binding to claudin-5. Cell. Mol. Life Sci. 72(7): 1417-1432 [PubMed] [WebPage] [PDF]
Rossa J, Plöger C, Vorreiter F, Saleh T, Protze J, Günzel D, Wolburg H, Krause G, Piontek J (2014) Claudin-3 and claudin-5 protein folding and assembly into the tight junction are controlled by non-conserved residues in transmembrane2 (TM3) and extracellular loop 2 (ECL2) segments. J. Biol. Chem. 289(11): 7641-7653 [PubMed] [WebPage] [PDF] [Supplements]
Rossa J*, Protze J* (*shared first authorship), Kern C, Piontek A, Günzel D, Krause G*, Piontek J* (*shared last authorship) (2014) Molecular and structural transmembrane determinants critical for embedding claudin-5 into tight junctions reveal distinct four helix bundle arrangement. Biochem. J. 464(1): 49-60 [PubMed] [WebPage] [PDF]
![]()
Molecular structure of tricellulin and arrangement within the tight junction
The crystal structure of tricellulin and its molecular architecture within the tricellular tight junction is not yet resolved.
In cooperation with the MDC, we have analyzed part of the protein, the C-terminal domain.
Schütz A, Radusheva V, Krug SM*, Heinemann U* (*shared last authorship) (2017) Crystal structure of the tricellulin C-terminal coiled-coil domain reveals a unique mode of dimerization. Ann. N.Y. Acad. Sci. 1405: 147-159
Westphal JK, Dörfel MJ, Krug SM, Cording JD, Piontek J, Blasig IE, Tauber R, Fromm M, Huber O (2010) Tricellulin forms homomeric and heteromeric tight junctional complexes. Cell. Mol. Life Sci. 67(12): 2057-2068 [PubMed] [WebPage] [Supplement WebPage] [PDF] [Suppl. Fig. 1] [Suppl. Fig. 2]
![]()
|
Barriers and channels formed by TJ proteins
This passage is cited from Krug et al. 2014: "Epithelia form barriers against unlimited passage of solutes and water, but also regulate and allow distinct permeation across that barrier. On the one hand, such permeation sites are located within the cell membranes, forming a transcellular pathway via channels, carriers, and transporting ATPases. On the other hand, the paracellular pathway between the cells is sealed against uncontrolled passage by the TJ.
However, long before claudins and TAMPs were identified as constituents of the TJ it was demonstrated that the paracellular pathway of some, but not all, epithelia is permeable to small ions [Frömter & Diamond 1972]. This in mind, the concept of “leaky” and “tight epithelia” was born [Diamond 1974]: in leaky epithelia the paracellular pathway is more ion-conductive than the transcellular one. In intestine and nephron, leaky epithelia are typically found in proximal segments. Tight epithelia behave the other way around and in intestine and nephron they are present in distal segments.
While many TJ proteins indeed have barrier-forming properties, there are also several claudins forming charge- and/or size-selective paracellular channels. These channels are not crossing membranes as transmembranal channels do, but are orientated parallel to the lateral membranes allowing permeation through theTJ. They are formed by the extracellular loops of TJ proteins interacting with extracellular loops of TJ proteins located in the opposing cell membrane.
Often there are uncertainties whether the conductive claudins should be named channels or pores. Simply said, both is correct: the pore is one part of a channel.
A channel is the entity of a permeation site comprising
(i) a pore,
(ii) a narrow site that restricts access by size and shape (size selectivity),
(iii) a site that favors passage by charge or charge density (charge selectivity), and
(iv) a feature providing time-variant permeability changes (gating).
By definition, “selective for x” means that the permeability for x is higher than that for other substances or groups of substances. All channel-forming claudins exhibit at least one of the three types of selectivity: for cations (claudin-2, claudin-10b, claudin-15), for anions (claudin-10a, claudin-17) or for water (claudin-2).
Charge selectivity cannot be determined from transepithelial resistance (TER) but from dilution potential measurements. Here, charge selectivitiy is read out from the resulting ratio PNa/PCl. PNa>PCl indicates cation selectivity and PNa<PCl indicates anion selectivity [Günzel et al. 2010; Yu et al. 2009]. Ratio changes together with the calculated absolute permeabilities give information about the preference. Higher selectivity, as e.g. exclusively for sodium only can be found in some membrane channels like the epithelial sodium channel ENaC, but yet not for any TJ protein. Thus, TJ protein channels formers and also barrier formers exhibit substrate-specific transmissive properties.
Therefore, the term “permeability” is incomplete without relying to the analyzed substance(s) for which the TJ protein is transmissive. (Krug et al. 2014)"
Martínez-Perafán F, Fromm A, van der Veen RE, Waldow A, Lehmann M, Krug SM, Günzel D, Rosenthal R, Fromm M, Piontek J (2025) Effect of claudin-1 or -3 expression on cation and water channel properties of claudin-2.
Gonschior H, Schmied C, Van der Veen R, Eichhorst J, Himmerkus N, Piontek J, Günzel D, Bleich M, Furuse M, Haucke V, Lehmann M (2022) Nanoscale segregation of channel and barrier claudins enables paracellular ion flux.
Meoli L, Günzel D (2020) Channel functions of claudins in the organization of biological systems. BBA - Biomembranes 1862(9): 183344 (18 pages) (°IF 3.8) [Part of Special Volume "The vertebrate epithelial apical junctional complex", Hervé JC ed.] [PubMed] [WebPage] [PDF] [Suppl. Fig. S1]
Fromm M, Piontek J, Rosenthal R, Günzel D, Krug SM (2017) Tight junctions of the proximal tubule and their channel proteins. Pflügers Arch. 469: 877-887 [PubMed] [WebPage] [PDF] (Review)
Krug SM, Schulzke JD, Fromm M (2014) Tight junction, selective permeability, and related diseases. Semin. Cell Devel. Biol. 36: 166-176 [PubMed] [WebPage] [PDF] (Review)
Günzel D, Krug SM, Rosenthal R, Fromm M (2010) Biophysical methods to study tight junction permeability. Curr. Top. Membr. 65: 39-78 [Directory] [WebPage] [PDF] (Review / book chapter)
![]()
For many years there had been a dispute regarding the contribution and even existence of paracellular water transport. It was in 2010 when this dispute ended after we discovered that it is claudin-2 that forms a water channel (Rosenthal et al. 2010). We showed that the claudin-2-based pore is permable to cations (Amasheh et al. 2002) as well as to water (Rosenthal et al. 2017). However, the ion permeability of other claudins is not necessarily coupled to water permeability: the cation channel claudin-10b and the anion channel claudin-17 proved to be not water permeable. In 2019, we presented a second water channel, claudin-15, with distinct function compared to claudin-2 (Rosenthal et al. 2019).
Martínez-Perafán F, Fromm A, van der Veen RE, Waldow A, Lehmann M, Krug SM, Günzel D, Rosenthal R, Fromm M, Piontek J (2025) Effect of claudin-1 or -3 expression on cation and water channel properties of claudin-2.
Günzel D (2022) Is there a molecular basis for solvent drag in the renal proximal tubule? Pflügers Arch. Eur. J. Physiol. ###: ###-### (5 pages) doi: 10.1007/s00424-022-02773-w (Review, Perspective)
Ayala-Torres C, Krug SM, Rosenthal R*, Fromm M* (*shared last authorship) (2021) Angulin-1 (LSR) affects paracellular water transport, however only in tight epithelial cells. Int. J. Mol. Sci.
Rosenthal R, Günzel D, Piontek J, Krug SM, Ayala-Torres C, Hempel C, Theune D, Fromm M (2020) Claudin-15 forms a water channel through the tight junction
with distinct function compared to claudin-2. Acta Physiol. 228(1): e13334 (15 pages),
doi: 10.1111/apha.13334
- Editorial Commentary on this article: Alexander RT (2020) Claudin-15 is not a drag! Acta Physiol. 228(1): e13397,
doi: 10.1111/apha.13397
Ayala-Torres C, Krug SM, Schulzke JD, Rosenthal R*, Fromm M* (*shared last authorship) (2019) Tricellulin effect on paracellular water transport. Int. J. Mol. Sci. 220(22): 5700 (15 pages) doi: 10.1016/j.cellsig.2019.109358
Fromm M, Piontek J, Rosenthal R, Günzel D, Krug SM (2017) Tight junctions of the proximal tubule and their channel proteins. Pflügers Arch. 469(7-8): 877-887 [PubMed] [WebPage] [PDF] (Review)
Rosenthal R, Czichos C, Theune D, Günzel D, Schulzke JD, Fromm M (2017) Water channels and barriers formed by claudins. Ann. N.Y. Acad. Sci.
Rosenthal R, Günzel D, Krug SM, Schulzke JD, Fromm M, Yu ASL (2017) Claudin-2-mediated cation and water transport share a common pore.
Acta Physiol.
219(2): 521-536 [PubMed] [WebPage]
[PDF]
![]()
Kirschner N*, Rosenthal R* (*shared first authorship), Furuse M, Moll I, Fromm M, Brandner JM (2013) Contribution of tight junction proteins to ion,
macromolecule, and water barrier in keratinocytes. J. Invest. Dermatol. 133(5): 1161-1169 [PubMed]
[WebPage] [PDF] [Supplement] Krug SM, Günzel D, Conrad MP, Rosenthal R, Fromm A, Amasheh S, Schulzke JD, Fromm M (2012) Claudin-17 forms tight junction
channels with distinct anion selectivity. Cell. Mol. Life Sci. 69(16): 2765-2778 [PubMed] [WebPage]
[PDF] [Supplement] Rosenthal R, Fromm M (2014) Significant water absorption goes paracellular in kidney proximal tubules.
Am. J. Physiol. Renal Physiol. 306(1): F51-F52 [PubMed] [WebPage]
[PDF] (Editorial)
Rosenthal R, Milatz S, Krug SM, Oelrich B, Schulzke JD, Amasheh S, Günzel D, Fromm M (2010) Claudin-2, a component of the tight junction,
forms a paracellular water channel.
J. Cell Sci. 123(11): 1913-1921 [PubMed] [WebPage]
[PDF] [Supplement]
![]()
Doctoral thesis, medicine: Dr. med. Beibei Oelrich (2009) Entwicklung und Etablierung einer Methode zur Messung des epithelialen Wassertransports an Claudin-exprimierenden MDCK-Zellen. Magna cum laude
![]()
The tricellular tight junction (tTJ) is localized at contacts of three epithelial or endothelial cells. Here, the elements of adjacent bTJ strands join and extend in basal direction. Importantly, the tTJ forms a vertical central tube which is considered to be a structural weak point of the whole TJ network. Proteins found predominatly at the tTJ are tricellulin and the angulins.
Tricellulin plays a critical role for barrier formation against macromolecule passage. This means that at low abundance of tricellulin the passage of medium-sized and large molecules will facilitated in this region (Krug et al. 2009). Of course, if any pathway opens for large molecules also small molecules and ions would pass, and this becomes numerically relevant in tissues low in claudin ion channels, i.e. "tight" epithelia (Krug 2017, Ann. N.Y. Acad. Sci.). The opening of the tTJ may occur in an unwanted or an intended manner:
Unwanted opening of the tTJ: In first studies with human colon biopsies we have shown that tricellulin is downregulated in the inflammatory bowel disease (IBD) ulcerative colitis and the tTJ is opened (Krug et al. 2017, Mucosal Immunol.). We hypothesize that this causes luminal pathogens to pass which then supports the inflammatory process (Krug et al. 2014). A role of other proteins, which are located at the tTJ, e.g. angulins (LSR, ILDR1, ILDR2), as well as for occludin might be assumed (Martini et al. 2017).
Intended opening of the tTJ: In a novel approach Masuo Kondoh and we developed a paracellular drug absorption enhancer acting at the tTJ, named angubindin-1. Its binding led to removal of angulin-1 and tricellulin from the tTJ which enhanced the permeation of macromolecular solutes (Krug et al. 2017, J. Contr. Release).
Weiß F, Czichos C, Knobe L, Voges L, Bojarski C, Michel G, Fromm M, Krug SM (2022) MarvelD3 is upregulated in ulcerative colitis and has attenuating effects during colitis indirectly stabilizing the intestinal barrier. Cells 11: 1551 (16 pages) doi: 10.3390/cells11091541
Dias MC, Quesada AO, Soldati S, Boesch F, Gruber I, Hildbrand T, Soenmez D, Khire T, Witz G, McGrath JL, Piontek J, Kondoh M, Deutsch U, Zuber B, Engelhardt B (2021) Brain endothelial tricellular junctions as novel sites for T-cell diapedesis across the blood-brain barrier. J. Cell Sci. 134(8): jcs253880, doi: org/10.1242/jcs.253880
Hu JCE, Bojarski C, Branchi F, Fromm M, Krug SM (2020) Leptin downregulates angulin-1 in active Crohn's disease via STAT3. Int. J. Mol. Sci.
Krug SM, Bojarski C, Fromm A, Lee IM, Dames P, Richter JF, Turner JR, Fromm M*, Schulzke JD* (*shared last authorship) (2018) Tricellulin is regulated via interleukin-13-receptor α2, affects macromolecule uptake, and is decreased in ulcerative colitis. Mucosal Immunol.
Krug SM, Hayaishi T, Iguchi D, Watari A, Takahashi A, Fromm M, Nagahama M, Takeda H, Okada Y, Sawasaki T, Doi T, Yagi K, Kondoh M (2017) Angubindin-1, a novel paracellular absorption enhancer acting at the tricellular tight junction. J. Contr. Release 260: 1-11 [PubMed] [WebPage] [PDF] [Supplement]
Krug SM (2017) Contribution of the tricellular tight junction to paracellular permeability in leaky and tight epithelia. Ann. N.Y. Acad. Sci. 1317(1): 219-230 [PubMed] [WebPage] [PDF]
Martini E, Krug SM, Siegmund B, Neurath MF, Becker C (2017) Mend your fences: The epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell. Mol. Gastroent. Hepatol. 4: 33-46
Krug SM, Schulzke JD, Fromm M (2014) Tight junction, selective permeability, and related diseases. Semin. Cell Devel. Biol. 36: 166-176 [PubMed] [WebPage] [PDF] (Review)
Krug SM, Amasheh S, Richter JF, Milatz S, Günzel D, Westphal JK, Huber O, Schulzke JD, Fromm M (2009) Tricellulin forms a barrier to
macromolecules in tricellular tight junctions without affecting ion permeability. Mol. Biol. Cell 20: 3713-3724 [PubMed]
[WebPage] [PDF] [Supplement
text] [Supplement video]
![]()
![]()
|
Intestine: Barrier defect in inflammatory bowel diseases (IBD)
Many diseases of the intestines are caused by impaired epithelial absorption or secretion and by impaired epithelial barrier function.
The pathogenesis of the ulcerative colitis and Crohn's disease is not fully understood so far. A typical symptom in both inflammatory bowel diseases is chronic diarrhea. We investigate the transport and barrier function of the intestine in vitro using two electrophysiological techniques, impedance spectroscopy and conductance scanning.
If the ion permeability is critically increased under pathological conditions a leak flux diarrhea occurs. This type of diarrhea is caused by massive fluxes of solutes and water from the blood into the gut lumen.
Regarding immunological mechanisms, an intact epithelial barrier keeps luminal bacteria, toxins, and antigens away from the subepithelial tissues. It is discussed, whether an impaired intestinal barrier allows for increased uptake of bacteria, toxins, and antigens which then will support the inflammation process.
Special Issue on IBD:
Krug SM, ed. (2024) Solving the puzzle: Molecular research in inflammatory bowel diseases, 2nd edition.
Special Issue of Int. J. Mol. Sci., Collection of papersKrug SM, ed. (2023) Solving the puzzle: Molecular research in inflammatory bowel diseases. Special Issue of Int. J. Mol. Sci. (12 papers) Collection of papers, doi: 10.3390/books978-3-0365-9863-5n
Reviews:
Liebing E*, Krug SM* (*shared first authorship), Neurath MF, Siegmund B, Becker C (2025) Wall of resilience: How the intestinal epithelium prevents inflammatory onslaught in the gut. Cell. Mol. Gastroenterol. Hepatol. 19(2): 101423 (17 pages), doi:10.1016/j.jcmgh.2024.101423
Angiocrine regulation of epithelial barrier integrity in inflammatory bowel disease
Martini E, Krug SM, Siegmund B, Neurath MF, Becker C (2017) Mend your fences: The epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell. Mol. Gastroent. Hepatol. 4(1): 33-46 [PubMed] [WebPage] [PDF]
Barmeyer C, Fromm M, Schulzke JD (2017) Active and passive involvement of claudins in the pathophysiology of intestinal inflammatory diseases. Pflügers Arch. 469(1): 15-26 (°IF 3.2) [PubMed] [WebPage] [PDF]
Barmeyer C, Schulzke JD, Fromm M (2015) Claudin-related intestinal diseases. Semin. Cell Devel. Biol. 42: 30-38 [PubMed] [WebPage] [PDF]
Bücker R, Schumann M, Amasheh S, Schulzke JD (2010) Claudins in intestinal function and disease. Curr. Top. Membr. 65: 195-227 [Directory] [WebPage] [PDF]
Bowel diseases
about which we have papers out:
- Crohns disease (Zeissig et al. 2004, Zeissig et al. 2007; Zeissig et al. 2008 see ENaC; Hu et al. 2020)
- Ulcerative colitis (Gitter et al. 2001; Heller et al. 2005; Krug et al. 2017; Kjærgaard S et al. 2020; Weiß et al. 2022)
- Collagenous colitis (Bürgel et a. 2002)
- Lymphocytic colitis (Barmeyer et al. 2017)
- Microscopic colitis (Barmeyer et al. 2012)
- Celiac disease (Schumann et al. 2017; Schumann Kamel et al. 2012; Schumann, Günzel et al. 2012; Schumann et al. 2017)
- Irritable bowel syndrome (Awad et al. 2023, Omarova et al. 2023)
- Inflamed pouch mucosa (Amasheh et al. 2009)
- HIV enteropathy (Epple et al. 2009; Epple et al. 2010; Krug et al. 2023)
- Whipple's disease (Epple et al. 2017)
- Graft-versus-host disease (Troeger et a. 2018)
- Cyanobacteria (Kaak et al. 2022)
Voges L (2025) Direct and indirect interaction mechanisms of immune cells and tight junction proteins. Doctoral Thesis, Dr. rer. nat., Freie Universität Berlin, magna cum laude
Bao LL, Yu YQ, González-Acera M, Patankar JV, Giessl A, Sturm G, Kühl AA, Atreya R, Erkert L, Gámez-Belmonte R, Krug SM, Schmid B, Tripal P, Chiriac M, Hildner K, Siegmund B, Wirtz S, Stürzl M, Abdou MM, Trajanoski Z, The TRR241 IBDome Consortium, Neurath MF, Zorzano A, Becker C (2025) Epithelial OPA1 links mitochondrial fusion to inflammatory bowel disease. Science Transl. Med. 17: eadn8699 (15 pages). doi: 10.1126/scitranslmed.adn8699
Voges L, Weiß F, Branco AT, Fromm M, Krug SM (2024) Expression and localization profiles of tight junction proteins in immune cells depend on their activation status. Int. J. Mol. Sci. 25(9): 4861 (18 pages), doi: 10.3390/ijms25094861
Amasheh S, Dullat S, Fromm M, Schulzke JD, Buhr HJ, Kroesen AJ (2009) Inflamed pouch mucosa possesses altered tight junctions indicating recurrence of inflammatory bowel disease. Int. J. Colorectal Dis. 24(10): 1149-1156 [PubMed] [WebPage] [PDF]
Barmeyer C, Erko I, Awad K, Fromm A, Bojarski C, Meissner S, Loddenkemper C, Kerick M, Siegmund B, Fromm M, Schweiger MR, Schulzke JD (2017) Epithelial barrier dysfunction in lymphocytic colitis through cytokine-dependent internalization of claudin-5 and -8. J. Gastroenterol. 52(10): 1090-1100 [PubMed] [WebPage] [PDF] [Supplement]
Barmeyer C, Erko I, Fromm A, Bojarski C, Allers C, Moos V, Zeitz M, Fromm M, Schulzke JD (2012) Ion transport and barrier function are disturbed in microscopic colitis. Ann. N.Y. Acad. Sci. 1258: 143-148 [PubMed] [WebPage] [PDF]
Bürgel N, Bojarski C, Mankertz J, Zeitz M, Fromm M, Schulzke JD (2002) Mechanisms of diarrhea in collagenous colitis. Gastroenterology 123(2): 433-443 [PubMed] [WebPage] [PDF]
Epple HJ, Allers K, Tröger H, Kühl A, Erben U, Fromm M, Zeitz M, Loddenkemper C*, Schulzke JD*, Schneider T* (*shared last authorship) (2010) Acute HIV infection induces mucosal infiltration with
CD4+ and CD8+ T cells, epithelial apoptosis, and a mucosal barrier defect. Gastroenterology
139(4): 1289-1300 [PubMed] [WebPage]
[PDF]
[Supplement PDF]
![]()
Epple HJ*, Friebel J*, Moos V* (*shared first authorship), Troeger H, Krug SM, Allers K, Schinnerling K, Fromm A, Siegmund B, Fromm M, Schulzke JD°, Schneider T° (°shared last authorship) (2017) Architectural and fuctional alterations of the small intestinal mucosa in classical Whipple's disease. Mucosal Immunol. 10(6): 1542-1552 [PubMed] [DOI] [PDF] [Supplement]
Epple HJ, Schneider T, Troeger H, Kunkel D, Allers K, Moos V, Amasheh M, Loddenkemper C, Fromm M, Zeitz M, Schulzke JD (2009) Impairment of the intestinal barrier is evident in untreated but absent in suppressively treated HIV-infected patients. Gut 58: 220-227 [PubMed] [WebPage] [PDF]
Gitter AH, Wullstein F, Fromm M, Schulzke JD (2001) Epithelial barrier defects in ulcerative colitis: characterization and quantification by electrophysiological imaging. Gastroenterology 121: 1320-1328 [PubMed] [WebPage] [PDF]
Heller F, Florian P, Bojarski C, Richter JF, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Bürgel N, Fromm M, Zeitz M, Fuss I, Strober W, Schulzke JD (2005) Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis and cell restitution. Gastroenterology 129(2): 550-564 [PubMed] [WebPage] [PDF]
Hu JCE, Bojarski C, Branchi F, Fromm M, Krug SM (2020) Leptin downregulates angulin-1 in active Crohn's disease via STAT3. Int. J. Mol. Sci.
Kaak JL, Lobo de Sá FD, Turner JR, Schulzke JD, Bücker R (2022) Unraveling the intestinal epithelial barrier in cyanotoxin microcystin-treated Caco-2 cell monolayers. Ann. NY Acad. Sci. 1516(1): 188-196, doi: 10.1111/nyas.14870
Kjærgaard S, Damm MMB, Chang J, Riis LB, Rasmussen HB, Hytting-Andreasen R, Krug SM, Schulzke JD, Bindslev N, Berner-Hansen M (2020) Altered structural expression and enzymatic activity parameters in quiescent ulcerative colitis: Are these potential normalization criteria? Int. J. Mol. Sci. (Special Issue "Update on Basic and Molecular Research in Inflammatory Bowel Disease") 21(5): e1887 (18 pages) [PubMed] [WebPage] [PDF]
Krug SM, Bojarski C, Fromm A, Lee IM, Dames P, Richter JF, Turner JR, Fromm M*, Schulzke JD* (*shared last authorship) (2018) Tricellulin is regulated via
interleukin-13-receptor α2, affects macromolecule uptake, and is decreased in ulcerative colitis.
Mucosal Immunol. 11(2): 345-356
[PubMed]
[WebPage]
[PDF]
[Supplement]
[Supplementary Movie] Meoli L, Günzel D (2023) The role of claudins in homeostasis. Nat. Rev. Nephrol.
(17 pages), doi: 10.1038/s41581-023-00731-y, free access:
https://rdcu.be/de2J0 (°IF 41.5)
Omarova S, Awad K, Moos V, Püning C, Gölz G, Schulzke JD, Bücker R (2023) Intestinal barrier in post-Campylobacter jejuni irritable bowel syndrome. Biomolecules 13(3): 449 (14 pages). doi: 10.3390/biom13030449
Troeger H, Hering NA, Bojarski C, Fromm A, Barmeyer C, Uharek L, Siegmund B, Fromm M, Rieger K*, Schulzke JD* (*shared last authorship) (2018) Epithelial barrier dysfunction as permissive pathomechanism in human intestinal graft-versus-host disease. Bone Marrow Transplant.
Promotion Dr. rer. nat. Franziska Weiß, "Interplay of the impaired tight junction and subjacent immune cells in inflammatory bowel diseases". Magna cum laude, Freie Universität Berlin, DFG-TRR 241-B6, supervison Susanne M. Krug
Weiß F, Czichos C, Knobe L, Voges L, Bojarski C, Michel G, Fromm M, Krug SM (2022) MarvelD3 is upregulated in ulcerative colitis and has attenuating effects during colitis indirectly stabilizing the intestinal barrier. Cells 11: 1551 (16 pages) doi: 10.3390/cells11091541
Zeissig S, Bürgel N, Günzel D, Richter JF, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD (2007) Changes in expression and distribution of claudin-2, -5 and -8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut 56(1): 61-72 [PubMed] [WebPage] [PDF]
Zeissig S, Bojarski C, Buergel N, Mankertz J, Zeitz M, Fromm M, Schulzke JD (2004) Downregulation of epithelial apoptosis and barrier repair in active Crohn's disease by TNFalpha antibody treatment. Gut 53: 1295-1302 [PubMed] [WebPage] [PDF]
Bacterial translocation through the intestinal wall has been studied under defined in vitro conditions in our lab.
Troeger H*, Richter JF* (*shared first authorship), Beutin L, Günzel D, Dobrindt U, Epple HJ, Gitter AH, Zeitz M, Fromm M, Schulzke JD (2007) E. coli alpha-hemolysin induces focal leaks in colonic epithelium – a novel mechanism of bacterial translocation. Cell. Microbiol. 9(10): 2530-2540 [PubMed] [WebPage] [PDF]
![]()
Awad K, Barmeyer C, Bojarski C, Nagel O, Lee IM, Schweiger MR, Schulzke JD*, Bücker R* (*shared last authorship) (2023) Epithelial barrier dysfunction in diarrhea-predominant irritable bowel syndrome (IBS-D) via downregulation of claudin-1. Cells 12(24), 2846 (17 pages). doi: 10.3390/cells12242846
Awad K, Barmeyer C, Bojarski C, Nagel O, Lee IM, Schweiger MR, Schulzke JD*, Bücker R* (*shared last authorship) (2023) Impaired intestinal permeability of tricellular tight junctions in patients with irritable bowel syndrome with mixed bowel habits (IBS-M). Cells 12(2), 236 (19 pages); doi: 10.3390/cells12020236, Supplement
Omarova S, Awad K, Moos V, Püning C, Gölz G, Schulzke JD, Bücker R (2023) Intestinal barrier in post-Campylobacter jejuni irritable bowel syndrome. Biomolecules 13(3): 449 (14 pages). doi: 10.3390/biom13030449
Cytokines like tumor necrosis factor alpha (TNFa), interleukins and interferons act as mediators of inflammation. In inflammatory bowel diseases (and in HIV infection) their local concentrations increase. We study the action of cytokines on transport and barrier function of human intestine and cell cultures originating from human colon (HT-29/B6).
Bücker R, ed. (2023) Special Issue "Enterotoxins and mucosal pathomechanisms", Special Issue of Toxins (Basel) (5 papers), Collection of papers
Cytokines and interleukins
about which we have papers out:
- TNFa (Schmitz et al. 1996; Schmitz et al. 1999; Barmeyer et al. 2004; Zeissig et al. 2004; Mankertz et
al. 2009; Amasheh et al. 2009; Amasheh et al. 2010)
- IFNg (Amasheh et al. 2009)
- IL-1β (Bode et al. 1998; Barmeyer et al. 2004)
- IL-2 (Barmeyer et al. 2004)
- IL-13 (Heller et al. 2005; Krug et al. 2018; Hader et al. 2023)
Amasheh M, Fromm A, Krug SM, Amasheh S, Andres S, Zeitz M, Fromm M, Schulzke JD (2010) TNFa-induced and berberine-antagonized tight junction barrier impairment via tyrosine kinase, pAkt, and NFkB signaling. J. Cell Sci. 123(23): 4145-4155 [PubMed] [WebPage] [PDF] [Supplements]
Amasheh M, Grotjohann I, Amasheh S, Fromm A, Söderholm JD, Zeitz M, Fromm M, Schulzke JD (2009) Regulation of mucosal structure and barrier function in rat colon exposed to tumor necrosis factor alpha and interferon gamma in vitro: A novel model for studying the pathomechanisms of inflammatory bowel disease cytokines. Scand. J. Gastroent. 44: 1226-1235 [PubMed] [WebPage] [PDF]
Amasheh S, Barmeyer C, Koch CS, Tavalali S, Mankertz J, Epple HJ, Gehring MM, Florian P, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD (2004) Cytokine-dependent transcriptional down-regulation of epithelial sodium channel (ENaC) in ulcerative colitis. Gastroenterology 126: 1711-1720 [PubMed] [WebPage] [PDF]
Barmeyer C*, Amasheh S* (*shared first authorship), Tavalali S, Mankertz J, Zeitz M, Fromm M, Schulzke JD (2004) IL-1beta and TNFalpha regulate sodium absorption in rat distal colon. Biochem. Biophys. Res. Comm. 317: 500-507 [PubMed] [WebPage] [PDF]
Barmeyer C, Harren M, Schmitz H, Heinzel-Pleines U, Mankertz J, Seidler U, Horak I, Wiedenmann B, Fromm M, Schulzke JD (2004) Mechanisms of diarrhea in the interleukin-2 deficient mouse model of colonic inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 286: G244–G252 [PubMed] [WebPage] [PDF]
Bode H, Schmitz H, Fromm M, Scholz P, Riecken EO, Schulzke JD (1998) IL1b and TNFa, but not IFNa, IFNg, IL6 or IL8, are secretory mediators in human distal colon. Cytokine 10: 457-465 [PubMed] [PDF]
Hader H
Heller F, Florian P, Bojarski C, Richter JF, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Bürgel N, Fromm M, Zeitz M, Fuss I, Strober W, Schulzke JD (2005) Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis and cell restitution. Gastroenterology 129(2): 550-564 [PubMed] [WebPage] [PDF]
Krug SM, Bojarski C, Fromm A, Lee IM, Dames P, Richter JF, Turner JR, Fromm M*, Schulzke JD* (*shared last authorship) (2018) Tricellulin is regulated via interleukin-13-receptor α2, affects macromolecule uptake, and is decreased in ulcerative colitis. Mucosal Immunol. 11(2): 345-356
Mankertz J*, Amasheh M* (*shared first authorship), Krug SM, Fromm A, Amasheh S, Hillenbrand B, Tavalali S, Fromm M, Schulzke JD (2009) Tumour necrosis factor alpha up-regulates claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol 3-kinase signaling. Cell Tiss. Res. 336(1): 67-77 [PubMed] [WebPage] [PDF]
Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ, Riecken EO, Schulzke JD (1999) Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology 116: 301-309. [PubMed] [WebPage] [PDF] [DCCV-Preis 1999 / award of the DCCV]
Schmitz H, Fromm M, Bentzel CJ, Scholz P, Detjen K, Mankertz J, Bode H, Epple HJ, Riecken EO, Schulzke JD (1999) Tumor necrosis factor-alpha (TNFa) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J. Cell Sci. 112: 137-146 [PubMed] [PDF]
Schmitz H, Fromm M, Bode H, Scholz P, Riecken EO, Schulzke JD (1996) Tumor necrosis factor alpha induces Cl– and K+ secretion in human distal colon driven by prostaglandin E2. Am. J. Physiol. 271: G669-G674 [PubMed] [PDF]
Zeissig S, Bojarski C, Buergel N, Mankertz J, Zeitz M, Fromm M, Schulzke JD (2004) Downregulation of epithelial apoptosis and barrier repair in active Crohn's disease by TNFalpha antibody treatment. Gut 53: 1295-1302 [PubMed] [WebPage] [PDF]
![]()
Intestine: Pathogens and tight junction
The lumen of the small and especially the large intestine is populated by an unimaginable
number of bacteria. Most are good-natured and help digesting food. Normally, they remain in the gut lumen and do not pass the intestinal wall, except the epithelial barrier is injured. However, some other bacteria are able to produce their own pathway across the gut wall. After wall passage they may act as pathogens, maintaining, enhancing or even initializing intestinal inflammation. The mechanisms by which pathogens can translocate are mainly paracellular, including (i) focal leaks, (ii) epithelial apoptosis, and (iii) opening the tight junction pathway, espectially at the tricellular tight junction.Bacteria, viruses, and toxins
about which we have papers out:
- Aeromonas hydrophila (Bücker et al. 2011)
- Aliarcobacter butzleri (formerly Arcobacter butzleri) (Karadas et al. 2015; Martins et al. 2022; Mateus et al. 2023)
- Arcobacter cryaerophilus (Bachus et al. 2025)
- Campylobacter jejuni (Bücker et al. 2018; Harrer et al. 2019, Nattramilarasu et al. 2020, Butkevych et al. 2020, Heimesaat et al. 2020, Lobo de Sá et al. 2021a, 20121b,
Omarova et al. 2023, Heimesaat et al. 2024)
- Cholera toxin (Markov et al. 2014)
- Clostridium difficile (Heils et al. 2023; Schneemann et al. 2023)
- Clostridium perfringens enterotoxin (Zhang et al. 2015; Protze et al. 2015; Liao et al. 2016; Eichner et al. 2018; Waldow et al. 2023, Nagarajan et al. 2024)
- Enterococcus faecium (Twardziok et al. 2014; Kern et al. 2017)
- Escherichia coli alpha-hemolysin (Bücker et al. 2014; Bücker et al. 2020; Schulz et al. 2021)
- Escherichia coli Nissle (Hering et al. 2014)
- Giardia duodenalis (Holthaus et al. 2021)
- Giardia lamblia (Troeger et al. 2007; Kraft et al. 2017; Hagen et al. 2025)
- Hantavirus (Witkowski et al. 2017)
- Norovirus (Troeger et al. 2009)
- Tick-borne encephalitis virus (Yu et al. 2014) (
Bücker R
Bücker R, Zakrzewski SS, Wiegand S, Pieper R, Fromm A, Fromm M, Günzel D, Schulzke JD (2020) Zinc prevents intestinal epithelial barrier dysfunction induced by alpha-hemolysin-producing Escherichia coli 536 infection in porcine colon. Vet. Microbiol. 243: 108632 (6 pages) [PubMed] [WebPage] [PDF] [Open link until May 08, 2020]
Bücker R, Krug SM, Fromm A,
Nielsen HL, Fromm M, Nielsen H, Schulzke JD (2017) Campylobacter fetus impairs barrier function in HT-29/B6 cells by focal tight junction alterations and leaks.
Bücker R, Krug SM, Moos V, Bojarski C, Schweiger MR, Kerick M, Fromm A, Janßen S, Fromm M, Hering NA, Siegmund B, Schneider T, Barmeyer C, Schulzke JD (2018) Diarrhoea of the Campylobacter jejuni colitis is due to malabsorption and epithelial leakage in the human host. Mucosal Immunol. 11(2): 474-485 [PubMed] [DOI] [PDF] [Supplement1] [Supplement2]
Bücker R, Krug SM, Rosenthal R, Günzel D, Fromm A, Zeitz M, Chakraborty T, Fromm M, Epple HJ, Schulzke JD (2011) Aerolysin from Aeromonas hydrophila perturbs tight junction integrity and cell lesion repair in intestinal epithelial HT-29/B6 cells. J. Infect. Dis. 204 (8): 1283-1292 [PubMed] [WebPage] [PDF]
Bücker R, Schulz E, Günzel D, Bojarski C, Lee IM, John LJ, Wiegand S, Janßen T, Wieler LH, Dobrindt U, Beutin L, Ewers C, Fromm M, Siegmund B, Troeger H, Schulzke JD (2014) α-Haemolysin of Escherichia coli: a potentiator of inflammatory activity in the colon. Gut 63(12): 1893-1901 [PubMed] [WebPage] [PDF] [Supplements]
Butkevych E, Lobo De Sá FD, Nattramilarasu PK, Bücker R (2020) Epithelial apoptosis and subepithelial immune responses in Campylobacter jejuni-induced barrier disruption Front. Microbiol. 11: 344 (14 pages) (°IF 4.3) [PubMed] [WebPage] [PDF]
Friebel J, Schinnerling K, Weigt K, Heldt C, Fromm A, Bojarski C, Siegmund B, Epple HJ, Kikhney J, Moter A, Schneider T, Schulzke JD, Moos V, Schumann M (2023) Uptake of Tropheryma whipplei by intestinal epithelia.
Harrer A, Bücker R, Boehm M, Zarzecka U, Tegtmeyer N, Sticht H, Schulzke JD, Backert S (2019) Campylobacter jejuni enters gut epithelial cells and impairs intestinal barrier function through cleavage of occludin by serine protease HtrA. Gut Pathog. 11: 4 (°IF 2.8) [PubMed] [WebPage] [PDF]
Heils L, Schneemann M, Gerhard R, Schulzke JD, Bücker R (2023) CDT of Clostridioides difficile induces MLC-dependent intestinal barrier dysfunction in HT-29/B6 epithelial cell monolayers. Toxins (Basel) 15(1): 54 (15 pages). doi: 10.3390/toxins15010054
Heimesaat MM, Mousavi S, Escher U, Lobo de Sá FD, Peh E, Schulzke JD, Kittler S, Bücker R, Bereswill S (2020) Resveratrol alleviates acute Campylobacter jejuni-induced enterocolitis in a preclinical murine intervention study. Microorganisms 8: 1858 (15 pages) [PubMed] [DOI] [PDF] [Supplement]
Heimesaat MM, Mousavi S, Lobo de Sá FD, Peh E, Schulzke JD, Bücker R, Kittler S, Bereswill S (2024) Oral curcumin ameliorates acute murine campylobacteriosis. Front Immunol. 15: 1363457 (14 pages). doi: 10.3389/fimmu.2024.1363457 (°IF 7.3)
Hering NA, Fromm A, Kikhney J, Lee IM, Moter A, Schulzke JD, Bücker R (2016) Yersinia enterocolitica affects intestinal barrier function in the colon. J. Infect. Dis. 213(7): 1157-1162 [PubMed] [WebPage] [PDF]
Hering NA, Luettig J, Krug SM, Wiegand S, Gross G, van Tol EA, Schulzke JD*, Rosenthal R* (*shared last authorship) (2017) Lactoferrin protects against intestinal inflammation or bacteria-induced barrier dysfunction in vitro. Ann. N.Y. Acad. Sci. 1405: 177-188 [PubMed] [WebPage] [PDF]
Hering NA*, Richter JF* (*shared first authorship), Fromm A, Wieser A, Hartmann S, Günzel D, Bücker R, Fromm M, Schulzke JD° (°corresponding author), Troeger H (2014) TcpC protein from E. coli Nissle improves epithelial barrier function involving PKCζ and ERK1/2 signalling in HT-29/B6 cells. Mucosal Immunol. 7(2): 369-378 [PubMed] [WebPage] [PDF]
Holthaus D*, Kraft MR* (*shared first authorship), Krug SM, Wolf S, Müller A, Delgado Betancourt E, Schorr M, Holland G, Knauf F, Schulzke JD, Aebischer T°, Klotz C° (°shared last authorship) (2022) Dissection of barrier dysfunction in organoid-derived human intestinal epithelia induced by Giardia duodenalis. Gastroenterology 162(3): 844-858, doi.org/10.1053/j.gastro.2021.11.022
Karadas G, Bücker R, Sharbati S, Schulzke JD, Gölz G (2015) Arcobacter butzleri isolates exhibit pathogenic potential in epithelial cell models, J. Appl. Microbiol. 120(1): 218-225 [PubMed] [WebPage] [Manuscript PDF]
Kern M, Günzel D, Aschenbach JR, Tedin K, Bondzio A, Lodemann U (2017) Altered cytokine expression and barrier properties after in vitro infection of porcine epithelial cells with enterotoxigenic Escherichia coli and probiotic Enterococcus faecium. Mediators Inflamm. 2017: 2748192 (13 pages) [PubMed] [WebPage] [PDF]
Liao Z, Yang Z, Piontek A, Eichner M, Krause G, Chen B, Li L, Piontek J, Zhang J (2016) Specific binding of a mutated fragment of Clostridium perfringens enterotoxin to endothelial claudin-5 and its modulation of cerebral vascular permeability. Neuroscience 327: 53-63 [PubMed] [WebPage] [PDF]
Lobo de Sá FD, Heimesaat MM, Bereswill S, Nattramilarasu PK, Schulzke JD, Bücker R (2021) Resveratrol prevents Campylobacter jejuni-induced leaky gut by restoring occludin and claudin-5 in the paracellular leak pathway.
Lobo de Sá FD, Schulzke JD, Bücker R (2021) Diarrheal mechanisms and the role of intestinal barrier dysfunction in Campylobacter infections. Curr. Top. Microbiol. Immunol. 431: 203-231. doi: 10.1007/978-3-030-65481-8_8
Markov AG, Falchuk EL, Kruglova NM, Rybalchenko OV, Fromm M, Amasheh S (2014) Comparative analysis of theophylline and cholera toxin in rat colon
reveals an induction of sealing tight junction proteins. Pflügers Arch. 466(11): 2059-2065 [PubMed]
[WebPage] [PDF] Martins R, Mateus C, Domingues F, Bücker R, Oleastro M, Ferreira S (2022) Effect of atmospheric conditions on pathogenic phenotypes of Arcobacter butzleri.
Mateus C, Maia CJ, Domingues F, Bücker R, Oleastro M, Ferreira S (2023) Evaluation of bile salts on the survival and modulation of virulence of
Aliarcobacter butzleri. Antibiotics (Basel)
12(9): 1387 (15 pages). doi: 10.3390/antibiotics12091387
Nattramilarasu PK, Bücker R, Lobo de Sá FD, Fromm A, Nagel O, Lee IM, Butkevych E, Mousavi S, Genger C, Kløve S, Heimesaat MM, Bereswill S, Schweiger MR, Nielsen HL, Troeger H, Schulzke JD (2020) Campylobacter concisus impairs sodium absorption in colonic epithelium via ENaC dysfunction and claudin-8 disruption. Int. J. Mol. Sci. (Special Issue "Ion and molecule transport in membrane systems 2.0") 21(2): e373 (23 pages) [PubMed] [WebPage] [PDF] [Supplements]
Omarova S, Awad K, Moos V, Püning C, Gölz G, Schulzke JD, Bücker R (2023) Intestinal barrier in post-Campylobacter jejuni irritable bowel syndrome. Biomolecules 13(3): 449 (14 pages). doi: 10.3390/biom13030449
Piontek A, Eichner M, Zwanziger D, Beier LS, Protze J, Walter W, Theurer S, Schmid KW, Führer-Sakel D, Piontek J*, Krause G* (*shared last authorship) (2019) Targeting claudin-overexpressing thyroid and lung cancer by modified Clostridium perfringens enterotoxin. Mol. Oncol. 14(2) :261-276 [PubMed] [WebPage] [PDF]
Protze J, Eichner M, Piontek A, Dinter S, Rossa J, Blecharz KG, Vajkoczy P, Piontek J*, Krause G* (*shared last authorship) (2015) Directed structural
modification of
Clostridium perfringens enterotoxin to enhance binding to claudin-5.
Cell. Mol. Life Sci. 72(7): 1417-1432 [PubMed] [WebPage]
[PDF]
Schneemann M, Heils L, Moos V, Weiß F, Krug SM, Weiner J, Beule D, Gerhard R, Schulzke JD, Bücker R (2023) A colonic organoid model challenged with
the large toxins of
Clostridioides difficile TcdA and TcdB exhibit deregulated tight junction proteins.
Toxins
15: 643 (19 pages). doi: 10.3390/toxins15110643
Schulz E, Schumann M, Schneemann M, Dony V, Fromm A, Nagel O, Schulzke JD, Bücker R (2021) Escherichia coli alpha-hemolysin HlyA induces host cell polarity changes, epithelial barrier dysfunction and cell detachment in human colon carcinoma Caco-2 cell model via PTEN-dependent dysregulation of cell junctions. Toxins 13(8): 520 (22 pages). doi: 10.3390/toxins13080520.
Troeger H, Epple HJ, Schneider T, Wahnschaffe U, Ullrich R, Burchard GD, Jelinek T, Zeitz M, Fromm M, Schulzke JD (2007) Effect of chronic Giardia
lamblia infection on epithelial transport and barrier function in human duodenum.
Troeger H, Loddenkemper C, Schneider T, Schreier E, Epple HJ, Zeitz M, Fromm M, Schulzke JD (2009) Structural and functional changes of the duodenum
in human norovirus infection. Gut 58: 1070-1077 [PubMed] [WebPage]
[PDF] Twardziok SO, Pieper R, {ABC order:} Aschenbach JR, Bednorz C, Brockmann GA, Fromm M, Klingspor S, Kreuzer S, Lodemann U, Martens H, Martin L,
Richter JF, Starke I, Siepert BF, Scharek-Tedin L, Tedin K, Vahjen W, Wieler LH, Zakrzewski SS, Zentek J, Wrede P (2014) Cross-talk between host, microbiome and probiotics: A systems biology
approach for analyzing the effects of probiotic
Enterococcus faecium NCIMB 10415 in piglets.
Waldow A*, Beier LS*
(*shared first authorship), Arndt J, Schallenberg S, Vollbrecht C, Bischoff P, Farrera-Sal M, Loch FN, Bojarski C, Schumann M, Winkler L, Kamphues C, Ehlen L°, Piontek J°
(°shared last authorship) (2023) cCPE fusion proteins as molecular probes to detect claudins and tight junction dysregulation in gastrointestinal cell lines, tissue explants and patient-derived
organoids. Pharmaceutics 15(7): 1980 (23 pages).
doi: 10.3390/pharmaceutics15071980 Whelan RA*, Rausch S* (*shared first authorship), Ebner F, Günzel D, Richter JF, Hering NA, Schulzke JD, Kühl AA, Keles A, Janczyk P, Nöckler K,
Wieler LH, Hartmann S (2014) A transgenic probiotic secreting a parasite immunomodulator for site-directed treatment of gut inflammation. Mol. Ther.
22(10): 1730-1740 [PubMed] [WebPage]
[PDF] Witkowski PT, Perley CC, Brocato RL, Hooper JW, Jürgensen C,
Schulzke JD, Krüger DH, Bücker R (2017) Gastrointestinal tract as entry route for hantavirus infection. Frontiers Microbiol.
8: 1721 [PubMed] [WebSite] [PDF] Yu C, Achazi K, Möller L,
Schulzke JD, Niedrig M, Bücker R (2014) Tick-borne encephalitis virus replication, intracellular trafficking, and pathogenicity in human intestinal Caco-2 cell
monolayers.
PLOS One
9(5): e96957 (print pages 1-10) [PubMed] [WebPage]
[PDF]
Zhang J*, Ni C*, Yang Z* (*shared first authorship), Piontek A, Chen H, Wang S, Fan Y, Qin Z, Piontek J (2015) Specific binding of Clostridium perfringens enterotoxin fragment 1 to claudin-b and modulation of zebrafish epidermal barrier. Exp. Dermatol.
![]()
Intestine: Diets, nutrients, and zinc
Diets or nutritional components
about which we have papers out:
Some components weaken the barrier:
- Gluten (Barmeyer et al. 2017)
- Nutrient deprivation (Demehri et al. 2016)
- Short-chain fatty acids (Meissner et al 2017; Georgi et al. 2014)
- Epigallocatechin-gallate (Rosenthal et al. 2025)
Zinc and other components are intended to act as intestinal barrier tighteners:
- Fermentable protein and fiber (Richter et al. 2014)
- Lactoferin (Hering et al. 2017)
- Myrrh extracts (Rosenthal et al. 2017)
- Shogaol (Luettig et al. 2016)
- Zinc
(Maares et al. 2018; Zakrzewski et al. 2017; Wiegand et al. 2017; Chai et al. 2014; Bücker et al. 2020)
Front. Pharmacol. 16: 1559812 (12 pages) doi: 10.3389/fphar.2025.1559812
Bücker R, Zakrzewski SS, Wiegand S, Pieper R, Fromm A, Fromm M, Günzel D, Schulzke JD (2020) Zinc prevents intestinal epithelial barrier dysfunction induced by alpha-hemolysin-producing Escherichia coli 536 infection in porcine colon. Vet. Microbiol. 243: 108632 (6 pages) [PubMed] [WebPage] [PDF] [Supplement]
Maares M, Keil C, Thomsen S, Günzel D, Wiesner B, Haase H (2018) Characterization of Caco-2 cells stably expressing the protein-based zinc probe eCalwy-5 as a model system for investigating intestinal zinc transport. J. Trace Elem. Med. Biol. 49: 296-304 [PubMed] [WebPage] [PDF]
Barmeyer C, Schumann M, Meyer T, Zielinski C, Zuberbier T, Siegmund B, Schulzke JD, Daum S, Ullrich R (2017) Long-term response to
gluten-free diet as evidence for non-celiac wheat sensitivity in one third of patients with diarrhea-dominant and mixed-type irritable bowel syndrome.
Int. J. Colorectal Dis.
32(1): 29-39 [PubMed] [WebPage] [PDF]
Chai W, Zakrzewski SS, Günzel D, Pieper R, Wang Z, Twardziok Z, Janczyk P, Osterrieder N, Burwinkel M (2014)
High-dose dietary zinc oxide mitigates infection with transmissible gastroenteritis virus in piglets.
BMC Vet. Res.
10(1): 75
[PubMed] [WebPage] [PDF]
[Supplement1] [Supplement2] Demehri FR*, Krug SM* (*shared first authorship), Feng Y, Lee IM, Schulzke JD, Teitelbaum DH (2016) Tight junction ultrastructure alterations in
a mouse model of enteral nutrient deprivation. Dig. Dis. Sci.
61(6): 1524-1533 [PubMed] [WebPage] [PDF] Georgi M, Rosendahl J, Ernst F, Günzel D, Aschenbach JR, Martens H, Stumpff F (2014) Epithelia of the ovine and bovine forestomach express basolateral
maxi-anion channels permeable to the anions of short chain fatty acids.
Pflügers Arch. 466(9):1689-712 [PubMed] [WebPage]
[PDF]
Hering NA, Luettig J, Krug SM, Wiegand S, Gross G, van Tol EA, Schulzke JD*, Rosenthal R* (*shared last authorship) (2017) Lactoferrin protects against intestinal inflammation or bacteria-induced barrier dysfunction in vitro. Ann. N.Y. Acad. Sci. 1405: 177-188 [PubMed] [WebPage] [PDF]
Luettig J, Rosenthal R, Lee IM, Krug SM, Schulzke JD (2016) The ginger component 6-shogaol prevents TNFα-induced barrier loss via inhibition of PI3K/Akt and NF-kB sigaling. Mol. Nutr. Food Res. 60(12): 2576-2586 [PubMed] [WebPage] [PDF]
Meissner S, Hagen F, Deiner C, Günzel D, Greco G, Shen Z, Aschenbach JR (2017) Key role of short chain fatty acids in epithelial barrier failure during ruminal acidosis. J. Dairy Sci. 100(8): 6662-6675 (°IF 2.4) [PubMed] [WebPage] [PDF]
Mudnakudu Nagaraju KK, Babina M, Weise C, Kühl A, Schulzke JD, Worm M (2016) Bortezomib treatment diminishes hazelnut-induced intestinal
anaphylaxis in mice.
Eur. J. Immunol. 46(7): 1727-1736 [PubMed] [WebPage]
[PDF] Richter JF, Pieper R, Zakrzewski SS, Günzel D, Schulzke JD, Van Kessel AG (2014) Diets high in fermentable protein and fiber alter tight junction
protein composition with minor effect on barrier function in piglet colon. Br. J. Nutr.
111(6): 1040-1049 [PubMed] [WebPage]
[PDF] [Supplements]
Rosenthal R, Luettig J, Hering NA, Krug SM, Albrecht U, Fromm M, Schulzke JD (2017) Myrrh exerts barrier-stabilizing and -protective effects in HT-29/B6 and Caco-2 intestinal epithelial cells. Int. J. Colorectal Dis. 32(5): 623-634 [PubMed] [WebPage] [PDF]
Wiegand S, Zakrzewski SS, Eichner M, Schulz E, Günzel D, Pieper R, Rosenthal R, Barmeyer C, Bleich A, Dobrindt U, Schulzke JD*, Bücker R* (*shared last authorship) (2017) Zinc treatment is efficient against Escherichia coli α haemolysin-induced intestinal leakage in mice. Sci. Rep. 7: 45649 (pages 1-13) [PubMed] [WebPage] [PDF]
![]()
Intestine: Celiac disease
In celiac disease, a T-cell-mediated response to gluten occurs in genetically predisposed individuals. Gluten is found in grains like wheat, barley, and rye. Importantly, gluten proteins contain peptide sequences which can elicit T-cell responses in the small intestine. This results in a malabsorptive enteropathy characterized by villus atrophy and crypt hyperplasia. The barrier defect includes altered tight junction proteins.
Schumann M, Siegmund B, Schulzke JD, Fromm M (2017) Celiac disease: role of the epithelial barrier. Cell. Mol. Gastroent. Hepatol.
Schumann M, Günzel D, Buergel N, Richter JF, Troeger H, May C, Fromm A, Sorgenfrei D, Daum S, Bojarski C, Heyman M, Zeitz M, Fromm M, Schulzke JD (2012) Cell polarity-determining proteins Par-3 and PP-1 are involved in epithelial tight junction defects in celiac disease. Gut 61(2): 220-228 [PubMed] [WebPage] [PDF] [Supplements]
Schumann M, Kamel S, Pahlitzsch ML, Lebenheim L, May C, Krauss M, Hummel M, Daum S, Fromm M, Schulzke JD (2012) Defective tight junctions in refractory celiac disease. Ann. N.Y. Acad. Sci. 1258: 43-51
Ménard S, Lebreton C, Schumann M, Matysiak-Budnik T, Dugave C, Bouhnik Y, Malamut G, Cellier C, Allez M, Crenn P, Schulzke JD, Cerf-Bensussan N, Heyman M (2012) Paracellular versus transcellular intestinal permeability to gliadin peptides in active celiac disease. Am. J. Pathol. 180(2): 608-615 [PubMed] [WebPage] [PDF]
![]()
Kidney
KlinPhysio publications focused on kidney: Listing
Pfeffer T*, Krug SM* (*shared first authorship), Kracke T, Schürfeld R, Colbatzky F, Kirschner P, Medert R, Freichel M, Schumacher D, Bartosova M, Zarogiannis SG, Muckenthaler MU, Altamura S, Pezer S, Volk N, Schwab C, Duensing S, Fleming T, Heidenreich E, Zschocke J, Hell R, Poschet G, Schmitt CP°, Peters V° (°shared last authorship) (2024) Knock-out of dipeptidase CN2 in human proximal tubular cells disrupts dipeptide and amino acid homeostasis and para- and transcellular solute transport. Acta Physiol. 240(4): e14126 (20 pages), doi: 10.1111/apha.14126
Meoli L, Günzel D (2023) The role of claudins in homeostasis. Nat. Rev. Nephrol.
Günzel D (2023) Is there a molecular basis for solvent drag in the renal proximal tubule?
Wuttke M, König E, Katsara MA, Kirsten H, Khomeijani Farahani S, Teumer A, Li Y, Lang M, Göçmen B, Pattaro C, Günzel D, Köttgen A, Fuchsberger C (2023) Imputation-powered whole-exome analysis identifies genes associated with kidney function and disease in the UK Biobank. Nat. Commun. 14: 1287 (16 pages), doi: 10.1038/s41467-023-36864-8
Kidney: FHHNC
(Familial Hypercalciuric Hypomagnesemia with NephroCalcinosis)In the thick ascending limb (TAL) of Henles loop, reabsorption of filtered NaCl takes place and generates a lumen-positive voltage. This voltage drives paracellular reabsorption of calcium and
magnesium. Disturbance of that reabsorption leads to renal Ca2+ and Mg2+ wasting, which occurs in patients with the rare inherited disorder of "Familial Hypercalciuric
Hypomagnesemia with NephroCalcinosis (FHHNC)".
Importantly, FHHNC has been found to be caused by mutations in two genes, coding for claudin-16 and
claudin-19, respectively. Functionally involved in these processes is also claudin-10b.
Kriuchkova N, Breiderhoff T, Müller D, Yilmaz DE, Demirci H, Drewell H, Günzel D, Himmerkus N, Bleich M, Persson PB, Mutig K (2023) Furosemide rescues hypercalciuria in familial hypomagnesaemia with hypercalciuria and nephrocalcinosis model. Acta Physiol. ###: ### (## pages), doi: 10.1111/apha.13927
Breiderhoff T*°, Himmerkus N* (shared first authorship), Drewell H, Plain A, Günzel D, Mutig K, Willnow TE, Müller D°, Bleich M° (°corresponding) (2017) Deletion of claudin-10 rescues claudin-16-deficient mice from hypomagnesmia and hypercalciuria. Kidney Int. 93(3): 580-588 [PubMed] [WebSite] [PDF]
Klar J, Piontek J, Milatz S, Tariq M, Jameel M, Breiderhoff T, Schuster J, Fatima A, Asif M, Sher M, Mäbert K, Fromm A, Baig SM, Günzel D, Dahl N (2017) Altered paracellular cation permeability due to a rare CLDN10B variant causes anhidrosis and kidney damage. Plos Genet.
Milatz S, Breiderhoff T (2017) One gene, two paracellular ion channels ‒ claudin-10 in the kidney. Pflügers Arch. 469(1): 115-121 [PubMed] [WebPage] [PDF] (Review)
Milatz S*, Himmerkus N* (*shared first authorship), Wulfmeyer VC, Drewell H, Mutig K, Hou J, Breiderhoff T, Müller D, Fromm M, Bleich M°, Günzel D° (°shared last authorship) (2017) Mosaic expression of claudins in thick ascending limbs of Henle results in spatial separation of paracellular Na+ and Mg2+ transport. Proc. Natl. Acad. Sci. USA 114(2): E219-E227 [PubMed] [WebPage] [PDF+Supplement]. "Paper of the month" 03/2017 of the German Physiological Society
Will C, Breiderhoff T, Thumfart J, Stuiver M, Kopplin K, Sommer K, Günzel D, Querfeld U, Meij IC, Shan Q, Bleich M, Willnow TE, Müller D (2010) Targeted deletion of murine Cldn16 identifies extra- and intrarenal compensatory mechanisms of Ca2+ and Mg2+ wasting. Am. J. Physiol. Renal Physiol. 298: F1152-F1161 [PubMed] [WebPage] [PDF]
Günzel D, Amasheh S, Pfaffenbach S, Richter JF, Kausalya PJ, Hunziker W, Fromm M (2009) Claudin-16 affects transcellular Cl- secretion in MDCK cells. J. Physiol. (Lond.) 587(15): 3777-3793 [PubMed] [WebPage] [PDF] [Supplement]
Kausalya PJ*, Amasheh S* (*shared first authorship, p 890), Günzel D, Wurps H, Müller D, Fromm M, Hunziker W (2006) Disease-associated mutations affect intracellular traffic and paracellular Mg2+ transport function of claudin-16. J. Clin. Invest. 116(4): 878-891
![]()
Kidney: HELIX syndrome (
Hypohidrosis, Electrolyte imbalance, Lacrimal gland dysfunction, Ichthyosis, and Xerostomia)HELIX syndrome is a rare genetically determined salt-losing renal tubulopathy. The acronym HELIX stands for Hypohidrosis, Electrolyte imbalance, Lacrimal gland dysfunction, Ichthyosis, and Xerostomia. Clinically, it is a non-Bartter, non-Gitelman disease characterized by altered handling of divalent cations and accompanied by dysfunctional salivary, sweat, and lacrimal glands. Sequence variants of CLDN10, which encode for claudin-10a and -10b, have been linked to that syndrome.
Sewerin S, Piontek J, Schönauer R, Grunewald S, Rauch A, Neuber S, Bergmann C, Günzel D*, Halbritter J* (*shared last authorship) (2022) Defective claudin-10 causes a novel variation of HELIX syndrome through compromised tight junction strand assembly. Genes & Dis. 9(5): 1301-1314. doi: 10.1016/j.gendis.2021.06.006
Alzahrani AS, Hussein M, Alswailem M, Mouna A, Albalawi L, Moria Y, Jabbar MA, Shi Y, Günzel D*, Dasouki M* (*shared last authorship) (2021) A novel claudin-10 mutation with a unique mechanism in two unrelated families with HELIX syndrome. Kidney Int. 100(2): 415-429, doi: 10.1016/j.kint.2021.02.023, Supplement
Hempel C, Protze J, Altun E, Riebe B, Piontek A, Fromm A, Lee IM, Saleh T, Günzel D, Krause G, Piontek J (2020) Assembly of tight junction strands: Claudin-10b and claudin-3 form homo-tetrameric building blocks that polymerise in a channel-independent manner. J. Mol. Biol. 432(7): 2405-2427 [PubMed] [WebPage] [PDF] [Supplementary Figures S1-S11] [Supplementary Table S1]
![]()
Cancers: Intestine, pancreas, oral mucosa, lung, nerve, prostate
Mousa SA*, Shaqura M* (*shared first authorship), Mohamed D, Hong X, Krug SM, Metwally EY, Assaf C, Treskatsch S, Schäfer M (2026) Tumor–macrophage–nerve interactions drive neuroinflammation and neuropathic pain in prostate cancer perineural invasion. Brain Behav. Immun. 133: 106280 (15 pages). doi: 10.1016/j.bbi.2026.106280
Sell T, Klotz C, Fischer MM, Astaburuaga-García R, Krug SM, Nitz M, Bu YJ, Drost J, Clevers H, Sers C, Morkel M, Blüthgen N (2023) Oncogenic signalling is coupled to colorectal cancer cell differentiation state. J. Cell Biol. ##: ###-### (accepted 07.03.2023, preprint doi: )
Zejc T, Piontek J, Schulzke JD, Fromm M, Ervens J*, Rosenthal R* (*shared last authorship) (2022) Clinical significance of claudin expression in oral squamous cell carcinoma. Int. J. Mol. Sci.
23(19): 11234 (13 pages), doi: 10.3390/ijms231911234Pahle J, Kobelt D, Aumann J, Behrens D, Daberkow O, Mokritzkij M, Piontek J, Stein U, Walther W (2021) Effective oncoleaking treatment of pancreatic cancer by claudin-targeted suicide gene therapy with Clostridium perfringens enterotoxin (CPE). Cancers 13(17): 4393 (26 pages). doi: 10.3390/cancers13174393, Supplement
Piontek A, Eichner M, Zwanziger D, Beier LS, Protze J, Walter W, Theurer S, Schmid KW, Führer-Sakel D, Piontek J*, Krause G* (*shared last authorship) (2019) Targeting claudin-overexpressing thyroid and lung cancer by modified Clostridium perfringens enterotoxin. Mol. Oncol. 14(2) :261-276 [PubMed] [WebPage] [PDF]
![]()
Neuro: Pain reduction in peripheral nerve
This research is mainly done in collaboration with Heike L. Rittner, Würzburg. One of the ideas behind is to modulate the tight junction of the perineurium for enhancing the access of peripheral analgetics.
Mousa SA*, Metwally EY* (*shared first authorship), Li X, Tafelski S, Retana Romero OA, Piontek J, Treskatsch S, Schäfer M, Shaqura M (2026) Human DRG glucocorticoid receptor profiling reveals targets for regionally delivered steroid analgesia.
Mousa SA*, Shaqura M* (*shared first authorship), Mohamed D, Hong X, Krug SM, Metwally EY, Assaf C, Treskatsch S, Schäfer M (2026) Tumor–macrophage–nerve interactions
drive neuroinflammation and neuropathic pain in prostate cancer perineural invasion. Brain Behav. Immun.
Atalla MS, Bettenhausen A-L, Verse JM, Cebulla N, Krug SM, Sauer R-S, Srivastava M, Bischler T, Chen JTC, Kortüm KM, Kittel RJ, Sommer C, Rittner HL (2025) Neuronal toxicity and recovery from early bortezomib-induced neuropathy: blood nerve barrier dysfunction without dorsal root ganglion damage. Br. J. Anaesth.S0007-0912(25)00366-6 (12 pages). doi: 10.1016/j.bja.2025.05.046
Krug SM*, Lee IM*, Knobe L* (*shared first authorship), Hartmannsberger B, Atalla MS, Rittner HL, Fromm M (2025) Characterising epi-perineurial barrier function by microscale techniques including a miniaturised Ussing chamber. Acta Biomater. 199: 290-300. doi: 10.1016/j.actbio.2025.04.043
Hartmannsberger B, Ben-Kraiem A, Kramer S, Guidolin C, Kazerani I, Doppler K, Thomas D, Gurke R, Sisignano M, Kalelkar PP, García AJ, Monje PV, Sammeth M, Nusrat A, Brack A, Krug SM, Sommer C, Rittner HL (2025) TAM receptors mediate the Fpr2-driven pain resolution and fibrinolysis after nerve injury. Acta Neuropathol. 149(1): 1 (21 pages). doi: 10.1007/s00401-024-02840-9 (°IF 9.3)
Reinhold AK, Krug SM, Salvador E, Sauer RS, Karl-Schöller F, Malcangio M, Sommer C, Rittner HL (2022) MicroRNA-21-5p functions via RECK/MMP9 as a proalgesic regulator of the blood nerve barrier in nerve injury. Ann. NY Acad. Sci. 1515(1): 184-195. doi: 10.1111/nyas.14816, TableS1, TableS2
Chen JTC, Schmidt L, Schürger C, Hankier MK, Krug SM, Rittner HL (2021) Netrin-1 as a multitarget barrier stabilizer in the peripheral nerve after injury. Int. J. Mol. Sci. (SI “Novel mechanisms and drug molecules modulating chronic pain") 22(18): 10090. doi.org/10.3390/ijms221810090, Supplement
Yang S*, Krug SM* (*shared first authorship), Heitmann J, Hu L, Reinhold AK, Sauer S, Bosten J, Sommer C, Fromm M, Brack A*, Rittner HL* (*shared last authorship) (2016) Analgesic drug delivery via recombinant tissue plasminogen activator and mRNA-183-triggered opening of the blood-nerve barrier. Biomaterials 82: 20-33 (IF 8.4) [PubMed] [WebPage] [PDF] [Supplement]
Sauer RS, Krug SM, Hackel D, Staat C, Konasin N, Yang S, Niedermirtl B, Bosten J, Günther R, Dabrowski S, Doppler K, Sommer C, Blasig IE, Brack A*, Rittner HL* (*shared last authorship) (2014) Safety, efficacy, and molecular mechanism of claudin-1-specific peptides to enhance blood-nerve-barrier permeability. J. Contr. Release 185: 88-98 (IF 7.7) [PubMed] [WebPage] [PDF] [Supplement]
Hackel D, Krug SM, Sauer RS, Mousa SA, Böcker A, Pflücke D, Wrede EJ, Kistner K, Hoffmann T, Niedermirtl B, Sommer C, Bloch L, Huber O, Blasig IE, Amasheh S, Reeh PW, Fromm M, Brack A, Rittner HL (2012) Transient opening of the perineurial barrier for analgesic drug delivery. Proc. Natl. Acad. Sci. USA 109(29): E2018-E2027 (IF 9.7) [PubMed] [WebPage] [PDF]
Rittner HL, Amasheh S, Moshourab R, Hackel D, Yamdeu RS, Fromm M, Stein C, Brack A (2012) Modulation of tight junction proteins in the perineurium to facilitate peripheral opioid analgesia. Anesthesiology 116(6): 1323-1334 (IF 5.2) [PubMed] [WebPage] [Full text via Ovid]
Hackel D, Brack A, Fromm M, Rittner HL (2012) Modulation of tight junction proteins in the perineurium for regional pain control. Ann. N.Y. Acad. Sci. 1257: 199-206 (IF 4.4) [PubMed] [WebPage] [PDF]
![]()
Skin: Epidermal barrier (incl. sweat gland)
This research is done in collaboration with Johanna Brandner, Hamburg. Human epidermis contains tight junctions the barrier function of which is investigated here.
Daci D*, Altrichter S* (*shared first authorship), Grillet F, Dib S, Mouna A, Suresh Kumar S, Terhorst-Molawi D, Maurer M, Günzel D°, Jörg Scheffel J° (°shared last authorship) (2024) Altered sweat composition due to changes in tight junction expression of sweat glands in cholinergic urticaria patients. Int. J. Mol. Sci
Beier LS*, Waldow A* (*shared first authorship), Khomeijani Farahani S, Mannweiler R, Vidal-Y-Sy S, Brandner JM, Piontek J°, Günzel D° (°shared last authorship) (2022) Claudin targeting as an effective tool for directed barrier modulation of the viable epidermis. Ann. NY Acad. Sci. 1517(1): 251-265, doi: 10.1111/nyas.14879
Wang Y, Scheffel J, Vera CA, Liu W, Günzel D, Terhorst-Molawi D, Maurer M, Altrichter S (2022) Impaired sweating in patients with cholinergic urticaria is linked to low expression of acetylcholine receptor CHRM3 and acetylcholine esterase in sweat glands.
Kirschner N*, Rosenthal R* (*shared first authorship), Furuse M, Moll I, Fromm M, Brandner JM (2013) Contribution of tight junction proteins to ion, macromolecule, and water barrier in keratinocytes. J. Invest. Dermatol. 133(5): 1161-1169 (IF 6.4) [PubMed] [WebPage] [PDF] [Supplement]. For this paper, authors gained the Heinz-Maurer Award for Dermatological Science 2014 (10 T€)
Kirschner N, Rosenthal R, Günzel D, Moll I, Brandner JM (2012) Tight junctions and differentiation – a chicken or the egg question? Exp. Dermatol. 21(3): 171-175 (IF 3.6) [PubMed] [WebPage] [PDF]
Kirschner N, Houdek P, Fromm M, Moll I, Brandner JM (2010) Tight junctions form a barrier in human epidermis. Eur. J. Cell Biol. 89(11): 839-842 [PubMed] [WebPage] [PDF]
![]()
Letizia M, Omar T, Weidner P, Jakob MO, Freise I, Krug SM, Löscher B-S, Rosati E, Obermayer B, Gamez-Belmonte R, Hecker J, Ziegler JF, Weixler B, Asbach P, Kunkel D, Stumvoll M, Miehle K, Becker C, Klose CSN, Glauben R, Beule D, Kühl AA, Conrad T, Tacke F, Wirtz S, Franke A, Sanders AD, Siegmund B, Weidinger C (2026) Characterization of intestinal immune responses in generalized human and murine lipodystrophy. J. Clin. Invest. (accepted 15.01.2026)
Marinovic I, Bartosova M, Levai E, Herzog R, Saleem A, Du Z, Zhang C, Sacnun JM, Pitaraki E, Sinis S, Damgov I, Krunic D, Lajqi T, Al-Saeedi M, Szabo JA, Hausmann M, Domonkos P, Kratochwill K, Krug SM, Zarogiannis SG, Schmitt CP (2025) Molecular and functional characterization of the peritoneal mesothelium, a barrier for solute transport. Function 6(1): zqae051 (12 pages), doi: 10.1093/function/zqae051
![]()
| Mechanisms |
|---|
Macromolecule permeation through the TJ
This passage is cited from Krug et al. 2014: Different paracellular pathways have been postulated for small ions and for macromolecules. Small ions predominantly pass through claudin-based channels of the bicellular TJ(bTJ). This pathway has been classified as a high-capacity, charge- and size-selective “pore pathway”. A lesser amount of small ions pass through the tricellular TJ (tTJ). Large molecules up to the size of macromolecules pass through strand discontinuities of the bTJ (Fig. 3B, left arrow). This pathway has been classified as a low-capacity, charge- and size-nonselective “leak pathway”. Another pathway for macromolecules could be provided by the tTJ. It is unknown to date which of the two pathways for macromolecules predominates.
Dias MC, Quesada AO, Soldati S, Boesch F, Gruber I, Hildbrand T, Soenmez D, Khire T, Witz G, McGrath JL, Piontek J, Kondoh M, Deutsch U, Zuber B, Engelhardt B (2021) Brain endothelial tricellular junctions as novel sites for T-cell diapedesis across the blood-brain barrier. J. Cell Sci. 134(8): jcs253880, doi: org/10.1242/jcs.253880
Krug SM, Schulzke JD, Fromm M (2014) Tight junction, selective permeability, and related diseases. Semin. Cell Devel. Biol. 36: 166-176 [PubMed] [WebPage] [PDF] (Review)
Krug SM, Bojarski C, Fromm A, Lee IM, Dames P, Richter JF, Turner JR, Fromm M*, Schulzke JD* (*shared last authorship) (2018) Tricellulin is regulated via interleukin-13-receptor α2, affects macromolecule uptake, and is decreased in ulcerative colitis. Mucosal Immunol. 11(2): 345-356
Krug SM (2017) Contribution of the tricellular tight junction to paracellular permeability in leaky and tight epithelia. Ann. N.Y. Acad. Sci. 1317(1): 219-230 [PubMed] [WebPage] [PDF]
Richter JF, Schmauder R, Krug SM, Gebert A, Schumann M (2016) A novel method for imaging sites of paracellular passage of macromolecules in epithelial sheets. J. Contr. Release 229: 70-79 [PubMed] [WebPage] [PDF] [Supplement] [Movie S1] [Movie S2]
Krug SM, Amasheh M, Dittmann I, Christoffel I, Fromm M, Amasheh S (2013) Sodium caprate as an enhancer of macromolecule permeation across tricellular tight junctions of intestinal cells. Biomaterials 34(1): 275-282 [PubMed] [WebPage] [PDF]
Krug SM, Amasheh S, Richter JF, Milatz S, Günzel D, Westphal JK, Huber O, Schulzke JD, Fromm M (2009) Tricellulin forms a barrier to
macromolecules in tricellular tight junctions without affecting ion permeability. Mol. Biol. Cell 20: 3713-3724 [PubMed]
[WebPage] [PDF] [Supplement
text] [Supplement video]
![]()
![]()
Drug absorption enhancers: temporary opening the TJ barrier
The epithelial wall of the intestine and the endothelial wall of the blood-brain barrier and the blood-nerve barrier, and other epithelial barriers produce a problem for many recently
developed drug molecules: they are not-lipophilic large molecules which cannot pass by diffusion through membrane channels or carriers. One modern general strategy to render drug passage possible is
to briefly and reversibly open the paracellular pathway.
Our research focuses on opening the tight junction at two sites: (i) the bicellular TJ by targeting claudins or (ii) the tricellular TJ by targeting
tricellulin and angulins. For review see here:
The barriers investgated are:
- Intestinal epithelium (Rosenthal et al. 2012; Takahashi et al. 2012; Krug et al. 2013; Dittmann et al. 2014; Krug et al. 2017; Eichner et al. 2017)
- Blood-brain barrier endothelium (Cording et al. 2017, Neuhaus et al. 2018)
- Perineurial blood-nerve barrier (Hackel et al. 2012; Sauer et al. 2014)
Neuhaus W, Piontek A, Protze J, Eichner M, Mahringer A, Subileau EA, Lee IM, Schulzke JD, Krause G, Piontek J (2018) Reversible opening of the blood-brain barrier by claudin-5-binding variants of Clostridium perfringens enterotoxin's claudin-binding domain. Biomaterials 161: 129-143 [PubMed] [WebPage] [PDF]
Krug SM, Hayaishi T, Iguchi D, Watari A, Takahashi A, Fromm M, Nagahama M, Takeda H, Okada Y, Sawasaki T, Doi T, Yagi K, Kondoh M (2017) Angubindin-1, a novel paracellular absorption enhancer acting at the tricellular tight junction. J. Contr. Release 260: 1-11 [PubMed] [WebPage] [PDF] [Supplement]
Cording J*, Arslan B* (*shared first authorship), Staat C, Dithmer S, Krug SM, Krüger A, Berndt P, Günther R, Winkler L, Blasig IE, Haseloff RF (2017) Trictide, a tricellulin-derived peptide to overcome cellular barriers. Ann. N.Y. Acad. Sci. 1405: 89-101
Sauer RS, Krug SM, Hackel D, Staat C, Konasin N, Yang S, Niedermirtl B, Bosten J, Günther R, Dabrowski S, Doppler K, Sommer C, Blasig IE, Brack A*, Rittner HL* (*shared last authorship) (2014) Safety, efficacy, and molecular mechanism of claudin-1-specific peptides to enhance blood-nerve-barrier permeability. J. Contr. Release 185: 88-98 [PubMed] [WebPage] [PDF] [Supplement]
Dittmann I, Amasheh M, Krug SM, Markov AG, Fromm M, Amasheh S (2014) Laurate permeabilizes the paracellular pathway for small molecules in the intestinal epithelial cell model HT-29/B6 via opening the tight junctions by reversible relocation of claudin-5. Pharm. Res. 31(9): 2539-2548 {Erratum correcting a misprint in the Title: Pharm. Res. 32(2): 737} [PubMed] [WebPage] [PDF]
Krug SM, Amasheh M, Dittmann I, Christoffel I, Fromm M, Amasheh S (2013) Sodium caprate as an enhancer of macromolecule permeation across tricellular tight junctions of intestinal cells. Biomaterials 34(1): 275-282 [PubMed] [WebPage] [PDF]
Hackel D, Krug SM, Sauer RS, Mousa SA, Böcker A, Pflücke D, Wrede EJ, Kistner K, Hoffmann T, Niedermirtl B, Sommer C, Bloch L, Huber O, Blasig IE, Amasheh S, Reeh PW, Fromm M, Brack A, Rittner HL (2012) Transient opening of the perineurial barrier for analgesic drug delivery. Proc. Natl. Acad. Sci. USA 109(29): E2018-E2027 [PubMed] [WebPage] [PDF]
Rosenthal R*, Günzel D* (*shared first authorship), Finger C, Krug SM, Richter JF, Schulzke JD, Fromm M, Amasheh S (2012) The effect of chitosan on transcellular and paracellular mechanisms in the intestinal epithelial barrier. Biomaterials 33(9): 2791-2800 [PubMed] [WebPage] [PDF] [Supplement]
Takahashi A*, Saito Y*, Kondoh M*°, Matsushita K, Krug SM, Suzuki H, Tsujino H, Li X, Aoyama H, Matsuhisa K, Uno T, Fromm M, Hamakubo T, Yagi K (2012) Creation and biochemical analysis of a broad-specific claudin binder. Biomaterials 33(12): 3464-3474 [PubMed] [WebPage] [PDF] (*shared first authorship, °corresponding author)
![]()
Current opinion assumes epithelial integrity during spontaneous apoptotic cell death. We measured, for the first time, the local conductances associated with apoptoses and show leaks of 50 nS (mean) in human intestinal epithelium. Measurements were performed employing the conductance scanning technique on confluent HT-29/B6 monolayers.
The results disprove the dogma that isolated cell apoptosis occurs without affecting the epithelial cell permeability barrier. After induction of apoptosis by tumor necrosis factor a (TNFa ) the apoptotic leaks were dramatically enhanced: not only increased the frequency 3fold, but the mean conductance increased 12fold to 600 nS. Thus apoptosis accounted for about half of the TNFa -induced permeability increase while the other half is caused by degradation of tight junctions in non-apoptotic areas.
Hence, spontaneous and, more so, induced apoptosis hollow out the intestinal barrier and may facilitate loss of solutes and uptake of noxious agents.
Krug SM, Grünhagen C, Allers K, Bojarski C, Seybold J, Schneider T, Schulzke JD, Epple HJ (2023) Macromolecule translocation across the intestinal mucosa of HIV-infected patients by transcytosis and through apoptotic leaks. Cells 12(14): 1887 (13 pages). doi: 10.3390/cells12141887
Schulzke JD, Bojarski C, Zeissig S, Heller F, Gitter AH, Fromm M (2006) Disrupted barrier function through epithelial cell apoptosis. Ann. N.Y. Acad. Sci. 1072: 288-299 [PubMed] [PDF] [Publisher's ad]
Heller F, Florian P, Bojarski C, Richter JF, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Bürgel N, Fromm M, Zeitz M, Fuss I, Strober W, Schulzke JD (2005) Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis and cell restitution. Gastroenterology 129(2): 550-564 [PubMed] [WebPage] [PDF]
Bojarski C, Weiske J, Schöneberg T, Schröder W, Mankertz J, Schulzke JD, Florian P, Fromm M, Tauber R, Huber O (2004) The specific fate of tight junction proteins in apoptotic epithelial cells. J. Cell Sci. 117: 2097-2107 [PubMed] [WebPage] [PDF]
Schmitz H, Rokos K, Florian P, Gitter AH, Fromm M, Scholz P, Ullrich R, Zeitz M, Pauli G, Schulzke JD (2002) Supernatants of HIV-infected immune cells affect the barrier function of human HT-29/B6 intestinal epithelial cells. AIDS 16(7): 983-991 [PubMed] [WebPage or PDF] [Related articles]
Bojarski C, Gitter AH, Bendfeldt K, Mankertz J, Schmitz H, Wagner S, Fromm M, Schulzke JD (2001) Permeability of HT-29/B6 colonic epithelium as a function of apoptosis. J. Physiol. (Lond.) 535(2): 541-552 [PubMed] [WebPage] [PDF]
Gitter AH, Bendfeldt K, Schulzke JD, Fromm M (2000) Leaks in the epithelial barrier caused by spontaneous and TNFa-induced single-cell apoptosis. FASEB J. 14(12): 1749-1753 [PubMed] [WebPage] [PDF]
![]()
Since the barrier function of an epithelium relies on its complete integrity, which is challenged by the loss of cells, by injury or apoptosis, rapid repair mechanisms are important. Already well known is the repair process of large epithelial wounds (restitution). The gap is closed by the immigration of intact adjacent cells within hours. Deep wounds require also cell proliferation, which takes several days. Little is known, however, about the - much faster - closure of single cell defects. Purely morphological studies cannot assess the functional leak that opens with the hiatus. Our new conductance scanning technique allows to measure the leak. Thus we investigate, for instance, the effects of inflammatory mediators on the restitution of single cell defects in epithelia of the intestine.
Günzel D*, Florian P* (*shared first authorship), Richter JF, Troeger H, Schulzke JD, Fromm M, Gitter AH (2006) Restitution of single-cell defects in the mouse colon epithelium differs from that of cultured cells. Am. J. Physiol. Reg. Integ. Comp. Physiol. 290: R1496-R1507 [PubMed] [WebPage] [PDF] [Supplemental videos]
Russo JM, Florian P, Shen L, Graham WV, Tretiakova MS, Gitter AH, Mrsny RJ, Turner JR (2005) Distinct temporal-spatial roles for rho kinase and myosin light chain kinase in epithelial purse-string wound closure. Gastroenterology 128(4): 987-1001 [PubMed] [WebPage] [PDF]
Florian P, Schöneberg T, Schulzke JD, Fromm M, Gitter AH (2002) Single-cell epithelial defects close rapidly by an actinomyosin purse string mechanism with functional tight junctions. J. Physiol. (Lond.) 545(2): 485-499 [PubMed] [WebPage] [PDF] [Supplementary movies]
![]()
 |
|
The conductance scanning technique in medium resolution allowed to measure in crypt and surface epithelium of the colon the
conductivities for Na+, K+, Cl–, which are defined by specific apical channels.
We demonstrated that cAMP-induced Cl–
secretion is localized not only - as assumed so far - in the crypts, but to a similar amount also in the surface epithelium:
The aldosterone-simulated electrogenic Na+ absorption is restricted to the surface epithelium:
Regarding the localization of the K+ secretion it is crucial how it is stimulated: The cAMP-induced K+ secretion is localized in crypts as well as in surface epithelium, while after stimulation by aldosterone K+ secretion is induced only in the surface epithelium:
![]()
The amiloride- blockable epithelial Na+ channel (ENaC) is an important protein for the regulation of the Na+ balance in vertebrates. It is a limiting factor for the absorption of Na+ in several organs. ENaC is regulated in epithelia of the distal kidney tubule and the distal colon by aldosterone and other corticosteroids.
The epithelial sodium channel (ENaC) was cloned a few years ago from intestinal epithelium of the rat [Canessa et al. 1993, Nature; Lingueglia et al. 1993, FEBS Lett.; Canessa et al. 1994, Nature]. The channel-forming protein consists of two a-, one b- and one g-subunit [Kosari et al. 1998, J. Biol. Chem.; Firsov et al. 1998, EMBO J.].
ENaC is regulated by aldosterone on the genomic level. The effect of aldosterone can be divided in 3 phases of: a latent phase, a phase of increasing Na+ transport, and a phase starting after 4 hours of stimulation of the basolateral Na+/K+-ATPase [Benos et al. 1995, J. Membr. Biol.]. The main component is the regulation of the epithelial Na+ channel: a blockade of ENaC by the diuretic amiloride stops the electrogenic Na+ transport (and the effect of aldosterone) completely [Benos 1982, Am. J. Physiol.].
We investigated electrogenic Na+ transport and, in identical tissues, mRNA expression of ENaC subunits in early (EDC) and late (LDC) distal colon of the rat. In both segments 8 h in vitro incubation with 3 nM aldosterone enhanced b- and gENaC mRNA and induced Na+ transport. Na+ transport was ten times higher in LDC than in EDC.
Nattramilarasu PK, Bücker R, Lobo de Sá FD, Fromm A, Nagel O, Lee IM, Butkevych E, Mousavi S, Genger C, Kløve S, Heimesaat MM, Bereswill S, Schweiger MR, Nielsen HL, Troeger H, Schulzke JD (2020) Campylobacter concisus impairs sodium absorption in colonic epithelium via ENaC dysfunction and claudin-8 disruption. Int. J. Mol. Sci. (Special Issue "Ion and molecule transport in membrane systems 2.0") 21(2): e373 (23 pages) (°IF 4.2) [PubMed] [WebPage] [PDF] [Supplements]
Dames P, Bergann T, Fromm A, Buecker R, Barmeyer C, Krug SM, Fromm M, Schulzke JD (2015) Interleukin-13 affects the intestinal epithelial sodium
channel (ENaC) by coordinated modulation of STAT6 and p38 MAPK activity>.
J. Physiol. (Lond.) 593(24): 5269-5282 [PubMed]
[WebPage] [PDF] Kuntzsch D, Bergann T, Dames P, Fromm A, Fromm M, Davis RA, Melzig MF, Schulzke JD (2012) The plant-derived glucocorticoid receptor agonist endiandrin
A acts as co-stimulator of colonic epithelial sodium channels (ENaC) via SGK-1 and MAPKs.
PLoS One 7(11): e49426 (print pages 1-14) [PubMed] [WebPage]
[PDF] Zeissig S, Bergann T, Fromm A, Bojarski C, Heller F, Guenther U, Zeitz M, Fromm M, Schulzke JD (2008) Altered ENaC expression leads
to impaired sodium absorption in the non-inflamed intestine in Crohn's disease. Gastroenterology
134(5): 1436-1447 (IF 12.6) [PubMed] [WebPage]
[PDF] Zeissig S, Fromm A, Mankertz J, Weiske J, Zeitz M, Fromm M, Schulzke JD (2007) Butyrate induces intestinal sodium absorption via
Sp3-mediated transcriptional up-regulation of epithelial sodium channels. Gastroenterology
132(1): 236-248 [PubMed] [WebPage+links]
[PDF] Zeissig S, Fromm A, Mankertz J, Zeitz M, Fromm M, Schulzke JD (2006) Restoration of ENaC expression by glucocorticoid receptor
transfection in human HT-29/B6 colon cells. Biochem. Biophys. Res. Commun.
344(4): 1065-1070 [PubMed] [WebPage]
[PDF] Grotjohann I, Schulzke JD, Fromm M (1999) Electrogenic Na+ transport in rat late distal colon by natural and synthetic
glucocorticosteroids and their metabolites. Am. J. Physiol. 276: G491-G498 [PubMed]
[WebPage] [PDF] Fromm M, Schulzke JD, Hegel U (1993) Control of electrogenic Na+ absorption in rat late distal colon by nanomolar
aldosterone added in vitro.
Am. J. Physiol. 264: E68-E73 [PubMed] [PDF]
Although it is known, that aldosterone regulates the electrogenic Na+ absorption via a transcriptional mechanism [Verrey & Beron 1996, NIPS], it was unresolved so far, whether aldosterone affects the electrogenic Na+ transport directly by de novo synthesis of channel proteins or indirectly by induction of regulatory proteins. We found that aENaC mRNA was unchanged in EDC, whereas it decreased in LDC. In LDC, b- and gENaC mRNA was induced 1 h after aldosterone addition whereas Na+ transport became apparent >1 h later. Down-regulation of aENaC does not take part in the acute regulation because it started after a lag time.
It was thought so far that the "early effect" of aldosterone is not based on genomic regulation, because ENaC transcription was reported to start later than Na+ transport. However, time correlation of b- and gENaC induction and Na+ transport stimulation suggests that the early aldosterone effect on Na+ absorption in distal colon may be due to transcriptional upregulation of b- and gENaC expression.
ENaC is induced by the mineralocorticoid rezeptor (MR) as well as by the glucocorticoid rezeptor (GR). ENaC expression is synergized by butyrate and by tumor necrosis factor-alpha (TNF-alpha).
Bergann T, Plöger S, Fromm A, Zeissig S, Borden SA, Fromm M, Schulzke JD (2009) A colonic mineralocorticoid receptor cell model expressing epithelial Na+ channels. Biochem. Biophys. Res. Comm. 381(2): 280-285 [PubMed] [WebPage] [PDF]
Bergann T, Zeissig S, Fromm A, Richter JF, Fromm M, Schulzke JD (2009) Glucocorticoid and tumor necrosis factor-alpha synergize to induce absorption by the epithelial sodium channel in the colon. Gastroenterology 136(3): 933-942 [PubMed] [WebPage] [PDF]
Ziera T, Irlbacher H, Fromm A, Latouche C, Krug SM, Fromm M, Jaisser F, Borden SA (2009) Cnksr3 is a direct mineralocorticoid receptor target gene and plays a key role in the regulation of the epithelial sodium channel. FASEB J. 23(11): 3936-3946 [PubMed] [WebPage] [PDF]
In inflammatory bowel diseases (IBD) ENaC expression and thus function is reduced. This effect is mediated by pro-inflammatory cytokines like interleukin-1beta and tumor necrosis factor-alpha:
Zeissig S, Bergann T, Fromm A, Bojarski C, Heller F, Guenther U, Zeitz M, Fromm M, Schulzke JD (2008) Altered ENaC expression leads to impaired sodium absorption in the non-inflamed intestine in Crohn's disease. Gastroenterology 134(5):1436-1447 [PubMed] [WebPage] [PDF]
Amasheh S, Barmeyer C, Koch CS, Tavalali S, Mankertz J, Epple HJ, Gehring MM, Florian P, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD (2004) Cytokine-dependent transcriptional down-regulation of epithelial sodium channel (ENaC) in ulcerative colitis. Gastroenterology 126: 1711-1720 [PubMed] [WebPage] [PDF]
Barmeyer C*, Amasheh S* (*shared first authorship), Tavalali S, Mankertz J, Zeitz M, Fromm M, Schulzke JD (2004) IL-1beta and TNFalpha regulate sodium absorption in rat distal colon. Biochem. Biophys. Res. Comm. 317: 500-507 [PubMed] [WebPage] [PDF]
Bürgel N, Bojarski C, Mankertz J, Zeitz M, Fromm M, Schulzke JD (2002) Mechanisms of diarrhea in collagenous colitis. Gastroenterology 123(2): 433-443 [PubMed] [WebPage] [PDF]
Barmeyer C, Horak I, Zeitz M, Fromm M, Schulzke JD (2003) The Interleukin-2-deficient mouse model. Pathobiology 70(3): 139-142 [PubMed] [Full text
Earlier papers on epithelial Na+ transport
Fromm M, Schulzke JD, Hegel U (1990) Aldosterone low dose, short term action in adrenalectomized glucocorticoid-substituted rats: Na, K, Cl, HCO3, osmolyte, and water transport in proximal and rectal colon. Pflügers Arch. 416: 573-579 [PubMed] [PDF]
Hierholzer K, Siebe H, Fromm M (1990) Inhibition of 11-b-hydroxysteroid dehydrogenase and its effect on epithelial sodium transport. Kidney Int. 38: 673-678 [Abstract+References] [PDF]
Fromm M, Hegel U (1987) Net ion fluxes and zero flux limiting concentrations in rat upper colon and rectum during anaesthesia-induced aldosterone liberation. Pflügers Arch. 408: 185-193 [PubMed] [PDF]
Fromm M, Oelkers W, Hegel U (1983) Time course of aldosterone and corticosterone plasma levels in rats during general anaesthesia and abdominal surgery. Pflügers Arch. 399: 249-254 [PubMed] [PDF]
Fromm M, Hegel U (1978) Segmental heterogeneity of epithelial transport in rat large intestine. Pflügers Arch. 378: 71-83 [PubMed] [PDF]
![]()
For a rare hereditary kidney disease causing calcium and magnesium deficency, see FHHNC.
Milatz S*, Himmerkus N* (*shared first authorship), Wulfmeyer VC, Drewell H, Mutig K, Hou J, Breiderhoff T, Müller D, Fromm M, Bleich M°, Günzel D° (°shared last authorship) (2016) Mosaic expression of claudins in thick ascending limbs of Henle results in spatial separation of paracellular Na
Gabriel TE, Günzel D (2007) Quantification of Mg2+ extrusion and cytosolic Mg2+-buffering in Xenopus oocytes. Arch. Biochem. Biophys. 458(1): 3-15 [PubMed] [WebPage] [PDF]
Günzel D, Kucharski LM, Kehres DG, Romero MF, Maguire ME (2006) The MgtC virulence factor of Salmonella enterica serovar typhimurium activates Na+,K+-ATPase. J. Bacteriol. 188(15): 5586-5594 [PubMed] [WebPage] [PDF]
Kausalya PJ*, Amasheh S* (*shared first authorship), Günzel D, Wurps H, Müller D, Fromm M, Hunziker W (2006) Disease-associated mutations
affect intracellular traffic and paracellular Mg2+
transport function of claudin-16. J. Clin. Invest.
116(4): 878-891
[PubMed] [WebPage]
[PDF]
![]()
Ebel H, Günther T (2006) Stimulation of choline/Mg2+ antiport in rat erythrocytes by mefloquine. Magnesium Res. 19(1): 7-11 [PubMed] [Full text]
Ebel H, Günther T (2005) Na+/Mg2+ antiport in erythrocytes of spontaneously hypertensive rats: role of Mg2+ in the pathogenesis of hypertension. Magnesium Res. 18(3): 175-185 [PubMed] [PDF]
Günzel D, Hintz K, Durry S, Schlue WR (2005) Mg2+-malate co-transport, a mechanism for Na+-independent Mg2+ transport in neurons of the leech Hirudo medicinalis. J. Neurophysiol. 94: 441-453 [PubMed] [WebPage] [PDF]
Günzel D, McGuigan JAS, Schlue WR (2005) Use of Mg2+ and Ca2+ macroelectrodes to measure binding in extracellular-like physiological solutions. Front. Biosci. 10: 905-918 [PubMed] [Full text] [Request text]
Ebel H, Hollstein M, Günther T (2004) Differential effect of imipramine and related compounds on Mg2+ efflux from rat erythrocytes. Biochim. Biophys. Acta - Biomembranes 1667(2):132-140 [PubMed] [WebPage] [PDF]
Ebel H, Kreis K, Günther T (2004) Regulation of Na+/Mg2+ antiport in rat erythrocytes. Biochim. Biophys. Acta - Biomembranes 1664(2): 150-160 [PubMed] [WebPage] [PDF]
Ebel H, Günther T (2003) Stimulation of Na+/Mg2+ antiport in rat erythrocytes by intracellular Cl-. FEBS Letters 543: 103-107 [PubMed] [WebPage] [PDF]
Müller A, Günzel D, Schlue WR (2003) Activation of AMPA/kainate receptors but not acetylcholine receptors causes Mg2+ influx into Retzius neurones of the leech Hirudo medicinalis. J. Gen. Physiol. 122: 727-739 [PubMed] [WebPage] [PDF]
Ebel H, Hollstein M, Günther T (2002) Role of the choline exchanger in Na+-independent Mg2+ efflux from rat erythrocytes. Biochim. Biophys. Acta - Biomembranes 1559(2): 135-144 [PubMed] [WebPage] [Full paper ,PDF]
![]()
SGLT1-mediated Na+-glucose transport
The interplay between membrane lipids and transport proteins is a significant, though not extesively investigated topic, in part due to the complexity of the methods. In our study by
Ebel et al. 2019 we found that membrane phospholipid composition alters Na+-dependent glucose transport by an acidic head group
which interferes with the transport function of SGLT1, by longer acyl chains which increase membrane thickness, and by unsaturated longer acyl chains which increase membrane fluidity.These findings
may lay the foundation for a general principle to attenuate dietary glucose uptake.
|
|
|
In most projects we alter epithelial transport or barrier properties in an electrophysiological experiment, and then use these functionally altered tissues for molecular biology. This are some less recent papers where these standard techniques are explained in more detail than in newer ones:
1. The epithelial sodium channel (ENaC)
Amasheh S, Barmeyer C, Koch CS, Tavalali S, Mankertz J, Epple HJ, Gehring MM, Florian P, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD (2004) Cytokine-dependent transcriptional down-regulation of epithelial sodium channel (ENaC) in ulcerative colitis. Gastroenterology 126: 1711-1720 [PubMed] [WebPage] [PDF]
Barmeyer C*, Amasheh S* (*shared first authorship), Tavalali S, Mankertz J, Zeitz M, Fromm M, Schulzke JD (2004) IL-1beta and TNFalpha regulate sodium absorption in rat distal colon. Biochem. Biophys. Res. Comm. 317: 500-507 [PubMed] [WebPage] [PDF]
Barmeyer C, Horak I, Zeitz M, Fromm M, Schulzke JD (2003) The Interleukin-2-deficient mouse model. Pathobiology 70(3): 139-142 [PubMed] [Full text]
Epple HJ, Amasheh S, Mankertz J, Goltz M, Schulzke JD, Fromm M (2000) Early aldosterone effect in distal colon by transcriptional regulation of ENaC subunits. Am. J. Physiol. 278(5): G718-G724 [PubMed] [WebPage] [PDF]
2. Tight junction proteins
Zeissig S, Bürgel N, Günzel D, Richter JF, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD (2007) Changes in expression and distribution of claudin-2, -5 and -8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut 56(1): 61-72 [PubMed] [WebPage] [PDF]
Zeissig S, Bojarski C, Bürgel N, Mankertz J, Zeitz M, Fromm M, Schulzke JD (2004) Downregulation of epithelial apoptosis and barrier repair in active Crohn's disease by TNFalpha antibody treatment. Gut 53: 1295-1302 [PubMed] [WebPage] [PDF]
Barmeyer C, Harren M, Schmitz H, Heinzel-Pleines U, Mankertz J, Seidler U, Horak I, Wiedenmann B, Fromm M, Schulzke JD (2004) Mechanisms of diarrhea in the interleukin-2 deficient mouse model of colonic inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 286: G244–G252 [PubMed] [WebPage] [PDF]
Bojarski C, Weiske J, Schöneberg T, Schröder W, Mankertz J, Schulzke JD, Florian P, Fromm M, Tauber R, Huber O (2004) The specific fate of tight junction proteins in apoptotic epithelial cells. J. Cell Sci. 117: 2097-2107 [PubMed] [WebPage] [PDF]
Mankertz J*, Hillenbrand B* (*shared first authorship), Tavalali S, Huber O, Fromm M, Schulzke JD (2004) Functional crosstalk between Wnt signaling and Cdx-related transcriptional activation in the regulation of the claudin-2 promoter activity. Biochem. Biophys. Res. Comm. 314(4): 1001-1007 [PubMed] [WebPage] [PDF]
Tebbe B, Mankertz J, Schwarz C, Amasheh S, Fromm M, Schultz-Ehrenburg U, Sánchez Ruderisch H, Schulzke JD, Orfanos CE (2002) Tight junction proteins: A novel class of integral membrane proteins. Expression in human epidermis and HaCaT keratinocytes. Arch. Dermatol. Res. 294: 14-18 [PubMed] [WebPage] [PDF]
Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, Fromm M (2002) Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 115(24): 4969-4976 [PubMed] [WebPage] [PDF]
Bürgel N, Bojarski C, Mankertz J, Zeitz M, Fromm M, Schulzke JD (2002) Mechanisms of diarrhea in collagenous colitis. Gastroenterology 123(2): 433-443 [PubMed] [WebPage] [PDF]
Mankertz J, Waller JS, Hillenbrand B, Tavalali S, Florian P, Schoneberg T, Fromm M, Schulzke JD (2002) Gene expression of the tight junction protein occludin includes differential splicing and alternative promoter usage. Biochem. Biophys. Res. Comm. 298: 657-666 [PubMed] [PDF]
Mankertz J, Tavalali S, Schmitz H, Mankertz A, Fromm M, Schulzke JD (2000) Expression from the human occludin promoter is affected by tumor necrosis factor a and interferon g. J. Cell Sci. 113(Pt 11): 2085-2090 [PubMed] [PDF] [GenBank: Homo sapiens occludin gene, partial sequence]
For the investigation of structural and functional characteristics we expose epithelial cell layers to proinflammatory cytokines under the standardised conditions of in vitro cell culture. During the incubation period the alteration of the transepithelial resistance is monitored. Subsequently, RNA and proteins are isolated from the cells, separated electrophoretically in a gel matrix and analyzed in Northern and Western blots with gene-specific probes or antisera against tight junction proteins.
Changes in quantity of the biomolecules permit conclusion on the regulation of gene expression and the role of the individual tight junction proteins. Beside classical hybridizing procedures we apply modern molecular biology methods for a structural and functional analysis of paracellular barrier and epithelial transport. With the genome walking technique it is possible to isolate regulatory sequences structurally linked to the gene-of-interest.
After nucleic acid sequencing, reporter gene analyses are performed to characterize these sequences functionally. Motives essential for transcription factor binding can be limited by targeted mutations. Thus, conclusions on the signal transduction cascades connected with the regulation of gene expression can be drawn. This is of importance for the development of improved therapeutic approaches of inflammatory bowel diseases.
![]()
Many molecules can be stained with an immunofluorescence dye and then detected or localized within the cell by confocal laser scanning microscopy (CLSM). Basic Example: By Western blotting Claudin-1 was found in the membrane fraction of MDCK-C11 cells, which is surprising, because MDCK-C11 form a rather leaky epithelium, while claudin-1 is typical for tight epithelia. However, claudin-1 was not colocalized with occludin within the tight junction but was found below the tight junction. Therefore it does not contribute to sealing properties of the tight junction (see Fig., from Amasheh et al., 2002, J Cell Sci.):
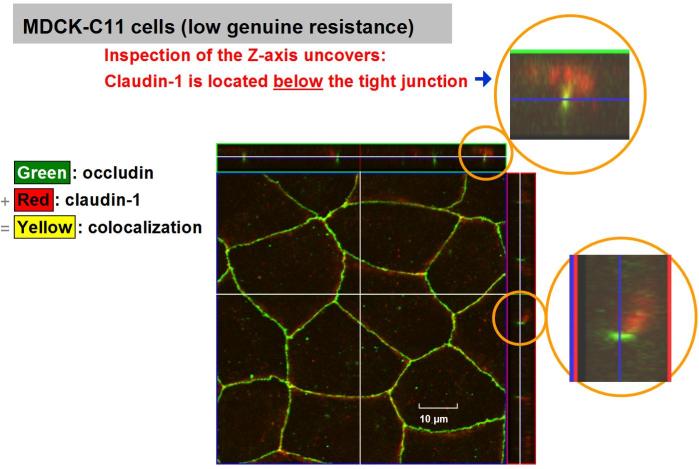
Amasheh S, Meiri N, Gitter AH, Schöneberg T, Mankertz J, Schulzke JD, Fromm M (2002) Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 115(24): 4969-4976 [PubMed] [WebPage] [PDF]
Mankertz J, Waller JS, Hillenbrand B, Tavalali S, Florian P, Schoneberg T, Fromm M, Schulzke JD (2002) Gene expression of the tight junction protein occludin includes differential splicing and alternative promoter usage. Biochem. Biophys. Res. Comm. 298: 657-666 [PubMed] [PDF]
Florian P, Schöneberg T, Schulzke JD, Fromm M, Gitter AH (2002) Single-cell epithelial defects close rapidly by an actinomyosin purse string mechanism with functional tight junctions. J. Physiol. (Lond.) 545(2): 485-499 [PubMed] [WebPage] [PDF] [Supplementary movies]
![]()
 Freeze-fracture electron microscopy of the tight junction
Freeze-fracture electron microscopy of the tight junctionPermeability of tight junctions are not determined solely by their molecular composition, but also - as known for many years - by the ultrastructural arrangement, expansion, and continuity of tight junction strands. The molecular composition and the ultrastructure are related to each other. Technically, tissues are freeze fractured, vaporized, analyzed electron microscopically. If the fracture occurs inside the lateral cell membrane often a tight junction meshwork becomme visible. In a morphometric analysis, tight junctions then are analyzed regarding the number of horizontally arranged strands, its extension, and its linearity.
Demehri FR*, Krug SM* (*shared first authorship), Feng Y, Lee IM, Schulzke JD, Teitelbaum DH (2016) Tight junction ultrastructure alterations in a mouse model of enteral nutrient deprivation. Dig. Dis. Sci.
61(6): 1524-1533 [PubMed] [WebPage] [PDF]Capaldo CT, Farkas AE, Hilgarth RS, Krug SM, Wolf MF, Benedik JK, Fromm M, Koval MH, Parkos CA, Nusrat A (2014) Proinflammatory cytokine-induced tight junction remodeling through dynamic self-assembly of claudins. Mol. Biol. Cell 25(18): 2710-2719 [PubMed] [WebPage] [PDF+Supplements]
Krug SM, Günzel D, Conrad MP, Rosenthal R, Fromm A, Amasheh S, Schulzke JD, Fromm M (2012) Claudin-17 forms tight junction channels with distinct anion selectivity. Cell. Mol. Life Sci. 69(16): 2765-2778 [PubMed] [WebPage] [PDF] [Supplement]
Milatz S, Krug SM, Rosenthal R, Günzel D, Müller D, Schulzke JD, Amasheh S*, Fromm M* (*shared last authorship) (2010) Claudin-3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes. Biochim. Biophys. Acta Biomembr. [PubMed] [WebPage] [PDF]
Rosenthal R, Milatz S, Krug SM, Oelrich B, Schulzke JD, Amasheh S, Günzel D, Fromm M (2010) Claudin-2, a component of the tight junction, forms a paracellular water channel. J. Cell Sci. 123(11): 1913-1921 [PubMed] [WebPage] [PDF] [Supplement]
Krug SM, Amasheh S, Richter JF, Milatz S, Günzel D, Westphal JK, Huber O, Schulzke JD, Fromm M (2009) Tricellulin forms a
barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol. Biol. Cell 20: 3713-3724 [PubMed]
[WebPage] [PDF] [Supplement
text] [Supplement video]
![]()
Zeissig S, Bürgel N, Günzel D, Richter JF, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD (2007) Changes in expression and distribution of claudin-2, -5 and -8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut 56(1): 61-72 [PubMed] [WebPage] [PDF]
Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ, Riecken EO, Schulzke JD (1999a) Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology 116: 301-309 [PubMed] [WebPage] [PDF]
Schmitz H, Fromm M, Bentzel CJ, Scholz P, Bode H, Epple HJ, Riecken EO, Schulzke JD (1999b) Tumor necrosis factor-alpha (TNFalpha) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J. Cell Sci. 112: 137-146 [PubMed] [PDF]
Schulzke JD, Bentzel CJ, Schulzke I, Riecken EO, Fromm M (1998) Epithelial tight junction structure in the jejunum of children with acute and treated celiac sprue. Pediatric Res. 43: 435-441 [PubMed] [WebPage]
Schulzke JD, Fromm M, Bentzel CJ, Zeitz M, Menge H, Riecken EO (1992) Ion transport in the experimental short bowel syndrome of the rat: Increased glucose-dependent Na-absorption is the main adaptive response. Gastroenterology 102: 497-504 [PubMed] [PDF]
Schulzke JD, Fromm M, Zeitz M, Menge H, Riecken EO, Bentzel CJ (1990) Tight junction regulation during impaired ion transport in blind loops of rat jejunum. Res. Exp. Med. 190: 59-68. [PubMed]
Schulzke JD, Fromm M, Bentzel CJ, Menge H, Riecken EO (1987) Adaptation of the jejunal mucosa in the experimental blind loop syndrome: changes in paracellular conductance and tight junction structure. Gut 28: 159-164 [PubMed] [PDF]
Bentzel CJ, Fromm M, Palant CE, Hegel U (1987) Protamine alters structure and conductance of Necturus gallbladder tight junctions without major electrical effects on the apical cell membrane. J. Membr. Biol. 95: 9-20 [PubMed] [PDF]
![]()
Isolated but "living" gastrointestinal epithelia and epithelial cell cultures can be functionally characterized regarding their transport and barrier functions by several electrophysiological methods.
Old fashioned, but still powerful in vitro technique for determination of active ion transport (short circuit current), transepithelial resistance (TER), and radioisotope fluxes. Details:
Fromm M, Schulzke JD, Hegel U (1993) Control of electrogenic Na
This technique was applied for many years also in the Lab course in Physiology for medical students an the Charité in Berlin. Tissues were distal colons obtained from a rural rabbit slaughtery close to Berlin. We have published this lab course:
Hegel U, Fromm M, Kreusel KM, Wiederholt M (1993) Bovine and porcine large intestine as model epithelia in a student lab course. Am. J. Physiol.
We have developed two refined techniques which allow for discrimination of local conductances within the epithelial tissue:
- Conductance scanning for the measurement of horizontal conductance distribution,
- Transmural impedance for the measurement of vertical conductance distribution.
Alija C, Knobe L,iourou I, Furuse M, Rosenthal R, Günzel D (2024) Integrating continuous transepithelial flux measurements into an Ussing chamber set-up. Int. J. Mol. Sci. 25(4): 2252 (13 pages), doi: 10.3390/ijms25042252
Krug SM*, Lee IM*, Knobe L* (*shared first authorship), Atalla MS, Rittner HL, Fromm M (2025) Characterization of the epi-perineurial barrier function using a novel Ussing chamber-based approach. Acta Biomater.
![]()
In order to describe quantitatively the heterogenous horizontal distribution of ion permeability in an epithelium, we developed a new electophysiological method. It was named conductance scanning, because it aims at the spatial resolution of epithelial conductivity by means of a scanning microelectrode probe.
The method is based upon the measurement of local differences in current density that are recorded with microelectrodes in the electrolyte solution above the mucosal surface of the flat epithelium, while a defined clamp current is passed through the tissue. Using mathematical models the distribution of epithelial conductivity is derived from the distribution of supraepithelial current density.
Günzel D, Krug SM, Rosenthal R, Fromm M (2010) Biophysical methods to study tight junction permeability. Curr. Top. Membr. 65: 39-78 [Directory] [WebPage] [PDF] (review / book chapter)
We have developed this method for 3 applications which differ reganding their spatial resolution and their mathematical models:
![]()
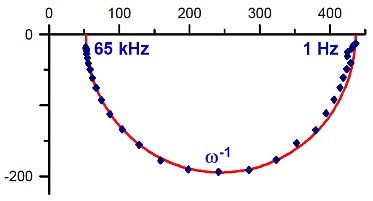 One-path impedance spectroscopy (1PI)
One-path impedance spectroscopy (1PI)Key word: Electrical impedance of epithelia
Discrimination of epithelial and subepithelial ion conductance in intact gastrointestinal epithelia in vitro. The conductance of the pure epithelium is frequency-dependent, however that of the tissues underlying the intestinal epithelium is not (in the frequency range applied). This is due to the fact that the subepithelial tissues have much higher conductivity due the lack of tight junctions. This allows to locate intestinal permeability changes to the epithelium or the subepithelium. Own development. Our first paper on 1PI: Fromm et al. 1985, J. Membr. Biol. [PubMed]; Fromm et al. 1985, Pflügers Arch. [PubMed].
By 1PI we have resolved why in inflammation intestine specimens conventional transepithelial resistance (TER) often did not significantly change: We found the resistance of the pure epithelium having clearly decreased while the resistance of the inflamed subepithelial tissues increased, in large part compensating the epithelial decrease. In vivo, the subepithelium does not contribute much to the barrier because capillary flow reaches until to the epithelial layer. In the Ussing chamber, of course, there is no blood flow and therefore the subepithelium adds to conventional TER (Review: Günzel et al. 2012 [PubMed]).
Günzel D, Zakrzewski S, Schmid T, Pangalos M, Wiedenhoeft J, Blasse C, Ozboda C, Krug SM (2012) From TER to trans- and paracellular resistance: Lessons from impedance spectroscopy. Ann. N.Y. Acad. Sci. 1257: 142-151 [PubMed] [WebPage] [PDF] (Review)
Epple HJ, Schneider T, Troeger H, Kunkel D, Allers K, Moos V, Amasheh M, Loddenkemper C, Fromm M, Zeitz M, Schulzke JD (2009) Impairment of the intestinal barrier is evident in untreated but absent in suppressively treated HIV-infected patients. Gut 58: 220-227 (Epub 04.11.2008) [PubMed] [WebPage] [PDF]
Gitter AH, Fromm M, Schulzke JD (1998) Impedance analysis for determination of epithelial and subepithelial resistance in intestinal tissues. J. Biochem. Biophys. Meth. 37: 35-46. [PubMed] [PDF]
Gitter AH, Schulzke JD, Sorgenfrei D, Fromm M (1997) Ussing chamber for high-frequency transmural impedance analysis of epithelial tissues. J. Biochem. Biophys. Meth. 35(2): 81-88. [PubMed] [WebPage] [PDF]
Mannweiler R, Bergmann S, Vidal-Y-Sy S, Brandner JM, Günzel D (2021) Direct assessment of individual skin barrier components by electrical impedance spectroscopy. Allergy 76(10): 3094-3106 (13 pages). doi: 10.1111/all.14851, Supplement PDF
Schulzke JD, Schulzke I, Fromm M, Riecken EO (1995) Epithelial barrier and ion transport in celiac sprue: electrical measurements on intestinal aspiration biopsies. Gut 37: 777-782. [PubMed] [Gut abstract+citations] [PDF]
Schulzke JD, Fromm M, Bentzel CJ, Zeitz M, Menge H, Riecken EO (1992) Ion transport in the experimental short bowel syndrome of the rat: increased glucose-dependent Na-absorption is the main adaptive response. Gastroenterology 102: 497-504. [PubMed] [PDF]
Schulzke JD, Fromm M, Hegel U (1986) Epithelial and subepithelial resistance of rat large intestine: Segmental differences, effect of stripping, time course, and action of aldosterone. Pflügers Arch. 407: 632-637 [PubMed] [PDF]
Fromm M, Palant CE, Bentzel CJ, Hegel U (1985) Protamine reversibly decreases paracellular cation permeability in Necturus gallbladder. J. Membr. Biol. 87: 141-150 [PubMed] [PDF]
Fromm M, Schulzke JD, Hegel U (1985) Epithelial and subepithelial contributions to transmural electrical resistance of intact rat jejunum, in vitro. Pflügers Arch. 405: 400-402 [PubMed] [PDF]
Two-path impedance spectroscopy (2PI)
This is a refined technique which is employed to determine paracellular and transcellular resistance in epithelial confluent celllayers. A specific
perturbation of one of the two pathways
allows for recording two data sets. Para- and transcellular resistance is calculated after combinig the two data sets and fluxes of a suitable paracellular marker.
Paper introducing 2PI: Krug et al. 2009, Biophys. J.[PubMed]
Method:
Schindler B, Günzel D, Schmid T (2021) Transcending two-path impedance spectroscopy with machine learning: A computational study on modeling and quantifying electric bipolarity of epithelia.
Schmid T, Bogdan M, Günzel D (2013) Discerning apical and basolateral properties of HT-29/B6 and IPEC-J2 cell layers by impedance spectroscopy, mathematical modeling and machine learning. PLOS One 8(7): e62913 (printed pages 1-12) [PubMed] [WebPage] [PDF] [Supplements, PDF]
Krug SM, Fromm M, Günzel D (2009) Two-path impedance spectroscopy for measuring paracellular and transcellular epithelial resistance. Biophys. J. 97(8): 2202-2211 [PubMed] [WebPage] [PDF] [Supplement]
Günzel D, Zakrzewski S, Schmid T, Pangalos M, Wiedenhoeft J, Blasse C, Ozboda C, Krug SM (2012) From TER to trans- and paracellular resistance: Lessons from impedance spectroscopy. Ann. N.Y. Acad. Sci. 1257: 142-151 [PubMed] [WebPage] [PDF] (Review)
Günzel D, Krug SM, Rosenthal R, Fromm M (2010) Biophysical methods to study tight junction permeability. Curr. Top. Membr. 65: 39-78 [Directory] [WebPage] [PDF] (review / book chapter)
Schmid T, Günzel D, Bogdan M (2010) Using an artificial neural network to determine electrical properties of epithelia. In: Diamantaras K, Duch W, Iliadis LS Eds., ICANN 2010, Part I, Lect. Notes Comput. Sci. 6352: 211-216 (DOI: 10.1007/978-3-642-15819-3_28) [WebPage] [PDF]
Application:
Krug SM, Amasheh M, Dittmann I, Christoffel I, Fromm M, Amasheh S (2013) Sodium caprate as an enhancer of macromolecule permeation across tricellular tight junctions of intestinal cells. Biomaterials 34(1): 275-282 [PubMed] [WebPage] [PDF]
Krug SM, Günzel D, Conrad MP, Rosenthal R, Fromm A, Amasheh S, Schulzke JD, Fromm M (2012) Claudin-17 forms tight junction channels with distinct anion selectivity. Cell. Mol. Life Sci. 69(16): 2765-2778 [PubMed] [WebPage] [PDF] [Supplement]
Amasheh M, Fromm A, Krug SM, Amasheh S, Andres S, Zeitz M, Fromm M, Schulzke JD (2010) TNFa-induced and berberine-antagonized tight junction barrier impairment via tyrosine kinase, pAkt, and NFkB signaling. J. Cell Sci. 123(23): 4145-4155 [PubMed] [WebPage] [PDF] [Supplements]
Milatz S, Krug SM, Rosenthal R, Günzel D, Müller D, Schulzke JD, Amasheh S*, Fromm M* (*shared last authorship) (2010) Claudin-3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes. Biochim. Biophys. Acta Biomembr. [PubMed] [WebPage] [PDF]
Krug SM, Amasheh S, Richter JF, Milatz S, Günzel D, Westphal JK, Huber O, Schulzke JD, Fromm M (2009) Tricellulin forms a barrier to macromolecules in tricellular tight junctions without affecting ion permeability. Mol. Biol. Cell 20: 3713-3724 [PubMed] [WebPage] [PDF] [Supplement text] [Supplement video]
Amasheh S*, Milatz S* (*shared first authorship), Krug SM, Bergs M, Amasheh M, Schulzke JD, Fromm M (2009) Na+ absorption defends from paracellular back-leakage by claudin-8 upregulation. Biochem. Biophys. Res. Comm. 378(1): 45-50 [PubMed] [WebPage] [PDF]
![]()
We develop organoid cultures of intestinal tissues mainly to thoroughly examine mechanistic aspects of inflammatory bowel diseases.
Organoid cultures originate from stem cells of crypts and develop combinations of several different cell types of a specific organ, in our case of the intestine. Intestinal organoids are more realistic models of "real life" than confluent flat intestinal cell lines consisting of only one cell type.
Mathew-Schmitt S, Oerter S, Reitenbach E, Gätzner S, Höchner A, Jahnke HG, Piontek J, Neuhaus W, Brachner A, Metzger M, Appelt-Menzel A (2025)
Schneemann M, Heils L, Moos V, Weiß F, Krug SM, Weiner J, Beule D, Gerhard R, Schulzke JD, Bücker R (2023) A colonic organoid model challenged with the large toxins of Clostridioides difficile TcdA and TcdB exhibit deregulated tight junction proteins. Toxins 15: 643 (19 pages). doi: 10.3390/toxins15110643
Waldow A*, Beier LS* (*shared first authorship), Arndt J, Schallenberg S, Vollbrecht C, Bischoff P, Farrera-Sal M, Loch FN, Bojarski C, Schumann M, Winkler L, Kamphues C, Ehlen L°, Piontek J° (°shared last authorship) (2023) cCPE fusion proteins as molecular probes to detect claudins and tight junction dysregulation in gastrointestinal cell lines, tissue explants and patient-derived organoids. Pharmaceutics 15(7): 1980 (23 pages). doi: 10.3390/pharmaceutics15071980
Weiß F, Holthaus D, Kraft M, Klotz C, Schneemann M, Schulzke JD, Krug SM (2022) Human duodenal organoid-derived monolayers serve as a suitable barrier model for duodenal tissue. Ann. NY Acad. Sci. 1515(1): 155-167, doi: 10.111/nyas.14804
Warschkau D, Delgado-Betancourt E, Holthaus D, Müller A, Kliem G, Krug SM, Schulzke JD, Aebischer T, Klotz C*, Seeber F* (*shared last authorship) (2022). From 3D to 2D: Harmonization of protocols for two-dimensional cultures on cell culture inserts of intestinal organoids from various species. Bio-protocol 12(2): e4295 (25 pages). doi: 10.21769/BioProtoc.4295
Krug SM, Schulzke JD, Project B06 "Interplay of the impaired tight junction and the subjacent immune cells in IBD", in: DFG Transregio Collaborative Research Center (TRR 241), coordinators Becker C (Erlangen) and Siegmund B (Berlin) "Immune-epithelial communication in inflammatory bowel diseases"
Holthaus D*, Kraft MR* (*shared first authorship), Krug SM, Wolf S, Müller A, Delgado Betancourt E, Schorr M, Holland G, Knauf F, Schulzke JD, Aebischer T°, Klotz C° (°shared last authorship) (2022) Dissection of barrier dysfunction in organoid-derived human intestinal epithelia induced by Giardia duodenalis. Gastroenterology 162(3): 844-858, doi.org/10.1053/j.gastro.2021.11.022
![]()
|
|
|
The highly differentiated cell line HT-29/B6 is a sub-clone of the human colon cancer cell line HT-29 [Kreusel et al., 1991].
Kreusel KM, Fromm M, Schulzke JD, Hegel U (1991) Cl– secretion in epithelial monolayers of mucus-forming human colon cells (HT-29/B6). Am. J. Physiol. 261: C574-C582. [PubMed] [PDF]
HT-29/B6 cells grow on permeable supports as epithelial monolayers. Secretagoges induce chloride secretion and mucus production [Epple et al., 1997]. They form apical brush borders and complete belts of tight junctions [Schmitz et al., 1999]. Consequently, they form epithelial barriers with properties of colon crypt cells [Gitter et al., 2000] and single cell apoptosis can be induced.[Bojarski et al., 2001].
The cell line HT-29/B6 is a versatile and well characterized model epithelium suitable for studying epithelial and/or intestinal properties with electrophysiological, morphological, and molecular methods.
Eichner M, Augustin C, Fromm A, Piontek A, Walther W, Bücker R, Fromm M, Krause G, Schulzke JD, Günzel D, Piontek J (2018) In colon epithelia, Clostridium perfringens enterotoxin causes focal leaks by targeting claudins which are apically accessible due to tight junction derangement. J. Infect. Dis. 217(1): 147-157 [PubMed] [WebPage] [PDF]
Bergann T, Plöger S, Fromm A, Zeissig S, Borden SA, Fromm M, Schulzke JD (2009) A colonic mineralocorticoid receptor cell model expressing epithelial Na+ channels. Biochem. Biophys. Res. Comm. 381(2): 280-285 [PubMed] [WebPage] [PDF]
Bergann T, Zeissig S, Fromm A, Richter JF, Fromm M, Schulzke JD (2009) Glucocorticoid and tumor necrosis factor-alpha synergize to induce absorption by the epithelial sodium channel in the colon. Gastroenterology 136(3): 933-942 [PubMed] [WebPage] [PDF]
Mankertz J*, Amasheh M* (*shared first authorship), Krug SM, Fromm A, Amasheh S, Hillenbrand B, Tavalali S, Fromm M, Schulzke JD (2009) Tumour necrosis factor alpha up-regulates claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol 3-kinase signaling. Cell Tiss. Res. 336(1): 67-77 [PubMed] [WebPage] [PDF]
Bojarski C, Gitter AH, Bendfeldt K, Mankertz J, Schmitz H, Wagner S, Fromm M, Schulzke JD (2001) Permeability of HT-29/B6 colonic epithelium as a function of apoptosis. J. Physiol. (Lond.) 535(2): 541-552 [PubMed] [PDF]
Gitter AH, Bendfeldt K, Schulzke JD, Fromm M (2000) Trans-/paracellular, surface/crypt, and epithelial/subepithelial resistances of mammalian colonic epithelia. Pflügers Arch. 439(4): 477-482 [PubMed] [PDF]
Schmitz H, Fromm M, Bentzel CJ, Scholz P, Detjen K, Mankertz J, Bode H, Epple HJ, Riecken EO, Schulzke JD (1999) Tumor necrosis factor-alpha (TNFa) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J. Cell Sci. 112: 137-146 [PubMed] [PDF]
Epple HJ, Kreusel KM, Hanski C, Schulzke JD, Riecken EO, Fromm M (1997) Differential stimulation of intestinal mucin secretion by cholera toxin and carbachol. Pflügers Arch. 433: 638-647 [PubMed] [PDF]
... and many more
![]()
There are two main inflammatory bowel diseases, ulcerative colitis and Crohn's disease. For excellent information on both diseases we refer to the German Crohn/Colitis society (DCCV) and the European Federation of Crohn's & Ulcerative Colitis Asscociations (EFCCA)
Martini E, Krug SM, Siegmund B, Neurath MF, Becker C (2017) Mend your fences: The epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell. Mol. Gastroent. Hepatol. 4: 33-46
Barmeyer C, Schulzke JD, Fromm M (2015) Claudin-related intestinal diseases. Semin. Cell Devel. Biol. 42: 30-38 [PubMed] [WebPage] [PDF] (Review)
Bücker R, Schulz E, Günzel D, Bojarski C, Lee IM, John LJ, Wiegand S, Janßen T, Wieler LH, Dobrindt U, Beutin L, Ewers C, Fromm M, Siegmund B, Troeger H, Schulzke JD (2014) α-Haemolysin of Escherichia coli: a potentiator of inflammatory activity in the colon. Gut 63(12): 1893-1901 [PubMed] [WebPage] [PDF] [Supplements]
Bücker R, Schumann M, Amasheh S, Schulzke JD (2010) Claudins in intestinal function and disease. Curr. Top. Membr. 65: 195-227 [Directory] [WebPage] [PDF] (review / book chapter)
![]()
Diarrhea can be driven by different mechanisms:
Leak flux diarrhea is caused by a break-down of the epithelial barrier, mostly produced by impaired tight junctions. This allows solutes and fluid to flow back into the gut lumen (=leak flux).
Zeissig S, Bürgel N, Günzel D, Richter JF, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD (2007) Changes in expression and distribution of claudin-2, -5 and -8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut 56(1): 61-72 [PubMed] [WebPage] [PDF]
Bürgel N, Bojarski C, Mankertz J, Zeitz M, Fromm M, Schulzke JD (2002) Mechanisms of diarrhea in collagenous colitis. Gastroenterology 123(2): 433-443 [PubMed] [WebPage] [PDF]
Bode H, Schmidt W, Schulzke JD, Fromm M, Zippel T, Wahnschaffe U, Bendfeldt K, Riecken EO, Ullrich R (2000) The HIV protease inhibitors saquinavir, ritonavir, and nelfinavir, but not indinavir, impair the epithelial barrier in the human intestinal cell line HT-29/B6. AIDS 13(18): 2595-2597 [PubMed reference] [Related articles]
Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ, Riecken EO, Schulzke JD (1999) Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology 116: 301-309.[PubMed] [WebPage] [PDF] [1999 award of the DCCV]
Schmitz H, Fromm M, Bentzel CJ, Scholz P, Detjen K, Mankertz J, Bode H, Epple HJ, Riecken EO, Schulzke JD (1999) Tumor necrosis factor-alpha (TNFa) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J. Cell Sci. 112: 137-146 [PubMed] [PDF]
Stockmann M, Fromm M, Schmitz H, Schmidt W, Riecken EO, Schulzke JD (1998) Duodenal biopsies of HIV infected patients with diarrhea show epithelial barrier defects but no secretion. AIDS 12: 43-51 [PubMed]
Stockmann M, Fromm M, Riecken EO, Schulzke JD (1998) Non-malabsorptive mechanisms of diarrhea in HIV infection. Pathobiology 66: 165-169 [PubMed] [PDF]
![]()
![]()
The Tight Junctions
Localization: Charité events & clubs in Berlin
Function: Live featuring classic rock
Clinical Impact: Animation of acoustically irradiated
hominids
Salah, Christian, Dirk, Roland, Susanne, Theresa (2001 - 2011)
![]()